Abstract
The neuronal isoform of nitric oxide synthase (nNOS) is highly expressed in mammalian skeletal muscle, but its functional role has not been defined. NO has been implicated in the local metabolic regulation of blood flow in contracting skeletal muscle in part by antagonizing sympathetic vasoconstriction. We therefore hypothesized that nNOS in skeletal muscle is the source of the NO mediating the inhibition of sympathetic vasoconstriction in contracting muscle. In the mdx mouse, a model of Duchenne muscular dystrophy in which dystrophin deficiency results in greatly reduced expression of nNOS in skeletal muscle, we found that the normal ability of skeletal muscle contraction to attenuate α-adrenergic vasoconstriction is defective. Similar results were obtained in mutant mice that lack the gene encoding nNOS. Together these data suggest a specific role for nNOS in the local metabolic inhibition of α-adrenergic vasoconstriction in active skeletal muscle.
To sustain physical activity, increases in skeletal muscle blood flow must closely match oxygen and substrate delivery to the increased metabolic rate of the contracting muscles. Although contracting skeletal muscle produces a number of vasodilator metabolites, the precise contributions of the various metabolites in mediating exercise-induced increases in muscle blood flow have been difficult to define.
One of the ways in which metabolites produced in contracting skeletal muscle may contribute to blood flow regulation during exercise is by opposing adrenergic vasoconstriction. An emerging body of evidence indicates that in mammalian skeletal muscle α-adrenergic vasoconstriction is very sensitive to inhibition by local metabolic products of skeletal muscle contraction (1–4). Although such metabolic modulation negates an otherwise deleterious effect of adrenergic vasoconstriction on muscle perfusion, little is known about the specific metabolites involved. NO may be one such metabolite, as suggested by increasing evidence demonstrating NO-mediated antagonism of α-adrenergic vasoconstriction both in isolated blood vessels and in intact vascular beds (5–10). Further support for the concept that NO is involved in the local metabolic modulation of adrenergic vasoconstriction is provided by a recent study in rats demonstrating that inhibition of NO synthase (NOS) enhances sympathetic vasoconstriction in the contracting hindlimb (11).
Until recently, the source of NO involved in muscle blood flow regulation during exercise was assumed to be the vascular endothelium, in which the endothelial isoform of NOS (eNOS) is abundantly expressed (12). In contracting muscle, the increased shear stress caused by elevated blood flow would seem to be an ideal stimulus to activate eNOS and increase endothelial NO production (13). However, the recent identification of the neuronal isoform of NOS (nNOS) in skeletal muscle provides another potential source of NO (14, 15). Conceivably, NO produced by nNOS in the skeletal muscle fibers could diffuse to nearby arterioles, resulting in vasodilation and increased blood flow. This blood flow control mechanism presumably would be engaged primarily during muscle contraction, in concert with Ca2+-dependent activation of nNOS. Accordingly, skeletal muscle-derived NO may function as a diffusable signaling molecule that couples the vascular tone of nutrient arterioles to the contractile activity of the skeletal muscle fibers in which they are embedded.
In normal skeletal muscle, the majority of nNOS is bound to the sarcolemma, where it exhibits a distribution similar to that of the cytoskeletal protein dystrophin (16, 17). Mutations in the dystrophin gene are the underlying cause of Duchenne muscular dystrophy (DMD) in humans (18, 19), which is a progressive and ultimately lethal disorder characterized by severe skeletal muscle degeneration. Recently, studies of dystrophin-deficient skeletal muscle of DMD patients and mdx mice, an animal model of DMD (20), have shown that the activity and expression of membrane-bound nNOS is greatly reduced (16, 17). Unlike human muscle in DMD, mdx muscle undergoes an early period of cell degeneration followed by regeneration such that adult animals typically display a mild phenotype (21). Despite this muscle regeneration, nNOS expression remains greatly reduced (17). This animal model of nNOS-deficient skeletal muscle therefore provided us with the opportunity to test the hypothesis that NO derived from nNOS modulates adrenergic vasoconstriction in contracting skeletal muscle. Using intact, anesthetized mice, we demonstrate that the normal ability of skeletal muscle contraction to attenuate α-adrenergic vasoconstriction is impaired in nNOS-deficient skeletal muscle of mdx mice. Likewise, modulation of α-adrenergic vasoconstriction is defective in contracting muscle of nNOS knockout mice, suggesting a specific antagonistic interaction between nNOS and α-adrenergic vasoconstriction in active skeletal muscle.
MATERIALS AND METHODS
Mice.
Male C57BL/10SnJ and mdx mice (23–34 g; The Jackson Laboratory) and male and female nNOS knockout mice (14–23 g) aged 10–14 weeks were used in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center. Generation of the nNOS knockout mice has been described (22).
Hemodynamic Measurements.
Mice were anesthetized with a mixture of Telazol and xylazine (7.5 mg/kg and 20 mg/kg, respectively, i.m.) followed by α-chloralose (80 mg/kg, i.v. with supplements as needed). Atropine sulfate (0.5 mg/kg, s.c.) was used to prevent excessive tracheal secretion. A catheter was placed in a jugular vein for drug infusions. Another catheter was placed in a carotid artery for the measurement of arterial blood pressure. The trachea was cannulated, and the mice were ventilated with room air and supplemental oxygen. Arterial blood gases were assessed at the end of each experiment (ABL-3; Radiometer, Copenhagen). Core temperature was maintained at 37°C with an external heat source. A Doppler crystal embedded in a soft silastic cuff was placed around the left femoral artery to measure changes in blood flow velocity by recording the pulsatile and mean Doppler shifts in kHz using a VF-1 pulsed Doppler flow system (Crystal Biotech, Holliston, MA). Changes in blood flow velocity as measured by Doppler shift signals previously have been established as reliable indicators of changes in volume flow (23). A catheter was placed in the right femoral artery and advanced to the abdominal aorta and was used for intraarterial hindlimb infusion of norepinephrine.
Hindlimb Contraction.
The left triceps surae muscles were connected to a force-displacement transducer (FT-10, Grass Instruments, Quincy, MA) via the calcaneal tendon. The left sciatic nerve was exposed, covered with warm mineral oil, and affixed to stimulating electrodes. To produce intermittent, tetanic contractions, the sciatic nerve was stimulated (model S88, Grass Instruments) at 2–3 times the motor threshold voltage with 100-ms trains of pulses (100 Hz, 0.2-ms duration) at a rate of 30 trains per min. Contraction periods of 10–20 min were separated by rest periods of at least 20 min.
In Vivo Assessement of Vasodilator and Vasoconstrictor Stimuli.
After baseline measurements of arterial blood pressure and femoral blood flow velocity were made, the hemodynamic responses to i.v. infusion of acetylcholine (3 μg/kg) or nitroprusside (0.8 μg/kg) were obtained. In addition, the hemodynamic responses to intraarterial hindlimb infusion of norepinephrine (3–9 ng in a volume of 2–7 μl) were obtained first when the hindlimb was at rest, and then during hindlimb contractions. Norepinephrine was infused during the contraction period when force had declined to ≈50% of the peak tension. The mice were then treated with N-nitro-l-arginine methyl ester (l-NAME; 10 mg/kg, i.v.), and these measurements were repeated.
Tissue Extraction and Western Blot Analysis.
Gastrocnemius muscles from C57 and mdx mice were excised, frozen in liquid nitrogen, and stored at −80°C for Western blot analysis of eNOS and nNOS. Muscles were homogenized in 20 vol (wt/vol) of buffer A [50 mM Tris⋅HCl, pH 7.5/1 μg/ml aprotinin/2 μg/ml leupeptin/20 μM tetrahydrobiopterin/0.1 mM DTT/1 μg/ml pepstatin A/10 μg/ml soybean trypsin inhibitor/1 mM benzamidine/1 mM EDTA/0.5 mM phenylmethylsulfonyl fluoride/20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)]. Homogenates were centrifuged for 10 min at 7,000 × g to separate the membrane pellet fractions from the cytosol fractions. Pellet fractions were resuspended in 20 vol of buffer A. Tissue extracts (20 μl) were subjected to SDS/PAGE 6% gel. Resolved proteins were transferred to nitrocellulose paper and subsequently probed for eNOS and nNOS. A mouse monoclonal anti-eNOS antibody (Transduction Laboratories, Lexington, KY) was used to detect eNOS, and a polyclonal antibody raised against the N terminus of nNOS was used to detect nNOS. Horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibodies were used to detect nNOS and eNOS bound antibodies, respectively. Immunoreactivity was detected by enhanced chemiluminescence (Amersham).
Data Analysis and Statistics.
Mean arterial blood pressures and mean femoral blood flow velocities (Doppler shifts) were measured from strip-chart recordings or digital files (MacLab, A. D. Instruments, Milford, MA). Femoral vascular conductance (kHz/mmHg; 1 mmHg = 133 Pa) was calculated as the mean Doppler shift (kHz) divided by mean arterial pressure (mmHg). The femoral blood flow velocity responses to norepinephrine and the arterial pressure responses to acetylcholine and nitroprusside were analyzed by measuring the peak decrease from baseline and the total area below baseline using the NIH image program on a Macintosh computer. Statistics were performed by using one-way or two-way analysis of variance with Scheffe’s post hoc test. Differences were considered statistically significant when P < 0.05. Results are expressed as mean ± SE.
RESULTS
eNOS Expression and Function Are Preserved in mdx Mice, Despite Reduced Neuronal NOS Expression.
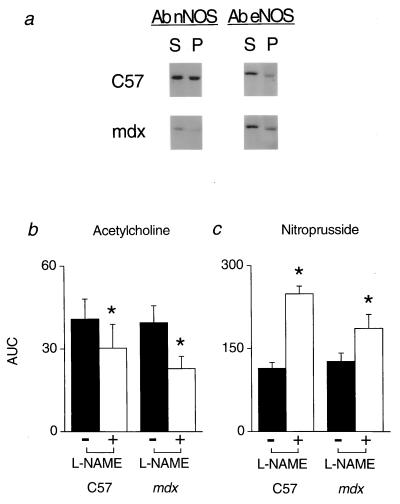
Because our purpose in studying the mdx mice was to evaluate the inhibitory role of NO derived from nNOS on adrenergic vasoconstriction in contracting skeletal muscle, it was first necessary to document that eNOS expression and function were not altered by nNOS-deficiency in the mdx mice. As expected, nNOS expression was markedly reduced in gastrocnemius muscle of mdx mice compared with C57 controls (Fig. 1a). In contrast, eNOS expression was comparable in muscle extracts from mdx and C57 mice (Fig. 1a).
Figure 1.
Expression of NOS isoforms in skeletal muscle and in vivo responses to endogenous and exogenous NO in C57 and mdx mice. (a) Western blots showing nNOS and eNOS immunoreactivity in supernatant (S) and pellet (P) fractions of gastrocnemius muscle homogenates. In mdx muscle, eNOS expression was normal, whereas nNOS expression was greatly reduced. Note that NOS immunoreactivity in the supernatant fractions is caused by the presence of the detergent CHAPS in the homogenizing buffer. Data shown are representative of results obtained in 2 C57 and 3 mdx mice. (b and c) Hypotensive responses to i.v. injection of the endogenous NO donor acetylcholine (3 μg/kg) or the exogenous NO donor nitroprusside (0.8 μg/kg) were similar in C57 (n = 8) and mdx (n = 8) mice (solid bars). In both groups of mice, NOS inhibition with l-NAME (open bars) attenuated the acetylcholine-mediated hypotension and enhanced the nitroprusside-mediated hypotension. AUC, area under the curve in arbitrary units; ∗, significantly different from response before l-NAME treatment.
Global eNOS function was evaluated in mdx and C57 mice by comparing in vivo hemodynamic responses to pharmacologic inhibition of NOS, which eliminates basal NO production, and the endothelium-dependent vasodilator acetylcholine, which stimulates NO production by activating eNOS. Anesthetized, artificially ventilated mdx and C57 mice had similar arterial Pco2 (36 ± 2 mmHg vs. 36 ± 2 mmHg), Po2 (190 ± 7 mmHg vs. 160 ± 10 mmHg), and pH (7.39 ± 0.03 vs. 7.38 ± 0.02). Baseline mean arterial blood pressures, femoral blood flow velocities, and vascular conductances also were similar in mdx and C57 mice (Table 1). Inhibition of NOS with l-NAME increased mean arterial pressure and decreased femoral blood flow velocity and vascular conductance comparably in mdx and C57 mice (Table 1). Before l-NAME treatment, intravenous injection of acetylcholine elicited similar hypotensive responses in mdx (−14 ± 2 mmHg) and C57 (−15 ± 2 mmHg) mice (Fig. 1b). In both groups of mice, this acetylcholine-mediated hypotension was attenuated comparably by l-NAME (Fig. 1b). Taken together, these data suggest that neither tonic nor agonist-stimulated eNOS activity differed in the mdx and C57 mice.
Table 1.
Hemodynamic responses to NOS inhibition in C57 and mdx mice
| Mean arterial pressure, mmHg
|
Femoral blood flow, kHz
|
Vascular conductance, kHz/mmHg
|
||||
|---|---|---|---|---|---|---|
| C57 | mdx | C57 | mdx | C57 | mdx | |
| Before l-NAME | 84 ± 2 | 84 ± 3 | 2.81 ± 0.32 | 2.02 ± 0.35 | 0.034 ± 0.004 | 0.025 ± 0.004 |
| After l-NAME | 129 ± 4 | 129 ± 5 | 1.29 ± 0.12 | 1.06 ± 0.16 | 0.010 ± 0.001 | 0.008 ± 0.001 |
| Δ | +44 ± 3* | +42 ± 4* | −1.65 ± 0.30* | −1.17 ± 0.29* | −0.025 ± 0.004* | −0.016 ± 0.003* |
Values are mean ± SE. C57, n = 19 before l-NAME, n = 17 after l-NAME; mdx, n = 18 before l-NAME, n = 14 after l-NAME. ∗, P < 0.05.
Hemodynamic Responses to an NO Donor Are Comparable in mdx and C57 Mice.
To determine whether mdx mice displayed altered sensitivity to the vasodilator effect of NO, the hemodynamic responses to infusion of the NO donor nitroprusside were measured. The hypotensive responses to nitroprusside were similar in mdx (−21 ± 3 mmHg) and C57 (−20 ± 1 mmHg) mice (Fig. 1c). l-NAME significantly enhanced nitroprusside-mediated hypotension in both groups of mice (Fig. 1c). This effect of NOS inhibition most likely was due to the development of supersensitivity to nitrovasodilators, which has been reported in rodents after removal of basal NO vasodilator tone (24).
α-Adrenergic Vasoconstriction Is Attenuated by NO Produced in Contracting C57 Mouse Hindlimb.
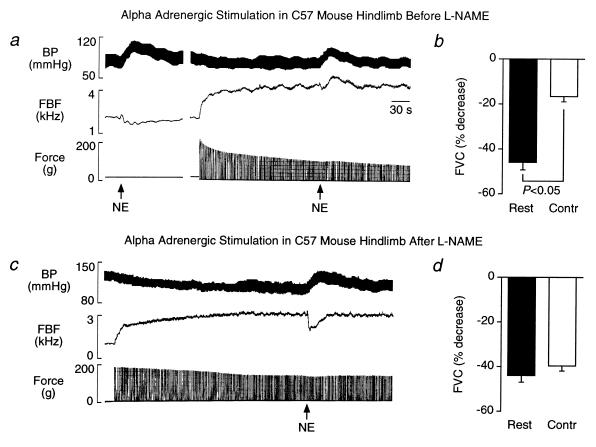
We previously showed (11) that α-adrenergic vasoconstriction is significantly attenuated by NO produced in contracting rat hindlimb. We now extend these results to mice by demonstrating that hindlimb contraction significantly attenuates norepinephrine-mediated vasoconstriction in C57 mice and that this effect of skeletal muscle contraction is reversed by NOS inhibition. Injection of norepinephrine into the arterial supply of the resting hindlimb increased blood pressure and decreased femoral blood flow velocity (−38 ± 4%) and vascular conductance (−46 ± 4%) (Fig. 2 a and b). As expected, hindlimb contraction alone increased femoral blood flow velocity and vascular conductance (Table 2). Compared with responses in resting hindlimbs, the vasoconstrictor responses to norepinephrine in contracting hindlimb were greatly attenuated (femoral blood flow velocity, −9 ± 3%; vascular conductance, −17 ± 2%) (Fig. 2 a and b).
Figure 2.
Metabolic inhibition of α-adrenergic vasoconstriction in contracting skeletal muscle is prevented by NOS inhibition in wild type C57 mice. (a and c) Segments of an original record from an experiment in one C57 mouse showing the arterial blood pressure (BP) and femoral blood flow velocity (FBF) responses to intraarterial injection of norepinephrine (NE) in resting and contracting hindlimbs before and after NOS inhibition with l-NAME. Before l-NAME treatment, NE injection in resting hindlimbs increased arterial pressure and decreased femoral blood flow velocity (Left in a). In contrast, NE-mediated vasoconstriction was attenuated in contracting hindlimbs, as demonstrated by an increase, rather than decrease, in femoral blood flow velocity in response to NE (Right in a). In this same mouse, l-NAME treatment prevented the contraction-induced inhibition of adrenergic vasoconstriction as indicated by the decrease in femoral blood flow velocity in response to NE during hindlimb contraction (c). (b and d) Summary data showing the NE-mediated decreases in calculated femoral vascular conductance (FVC) in resting and contracting (Contr) hindlimb before and after l-NAME treatment. Hindlimb contraction greatly attenuated the NE-mediated decrease in vascular conductance before (b; n = 10), but not after (d; n = 8), l-NAME treatment.
Table 2.
Hemodynamic responses to hindlimb contraction in C57, mdx, and nNOS (−/−) mice
| Mean arterial pressure, mmHg
|
Femoral blood flow, kHz
|
Vascular conductance, kHz/mmHg
|
Peak hindlimb force, g
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C57 | mdx | nNOS (−/−) | C57 | mdx | nNOS (−/−) | C57 | mdx | nNOS (−/−) | C57 | mdx | nNOS (−/−) | |
| Rest | 82 ± 3 | 81 ± 4 | 102 ± 5 | 1.89 ± 0.10 | 1.47 ± 0.11 | 1.04 ± 0.24 | 0.024 ± 0.022 | 0.019 ± 0.002 | 0.010 ± 0.002 | — | — | — |
| Contraction | 65 ± 3 | 66 ± 4 | 85 ± 6 | 2.72 ± 0.22 | 2.68 ± 0.20 | 1.81 ± 0.25 | 0.043 ± 0.004 | 0.041 ± 0.003 | 0.022 ± 0.003 | 156 ± 13 | 175 ± 17 | 140 ± 9 |
| Δ | −17 ± 2* | −15 ± 3* | −17 ± 3* | +0.83 ± 0.18* | +1.22 ± 0.15* | +0.78 ± 0.06* | +0.019 ± 0.003* | +0.023 ± 0.003* | +0.012 ± 0.001* | — | — | — |
Values are mean ± SE. C57, n = 10; mdx, n = 10, nNOS (−/−), n = 6. ∗, P < 0.05.
In resting hindlimb, NOS inhibition with l-NAME did not alter norepinephrine-mediated vasoconstriction (Fig. 2d). l-NAME did not affect the initial force produced by the contracting hindlimb (154 ± 15 g), but did decrease the rate of decline (t1/2 = 3.6 ± 0.3 min before vs. 7.3 ± 1.0 min after l-NAME treatment). l-NAME also did not affect the increases in femoral blood flow velocity (+1.02 ± 0.13 kHz) or vascular conductance (+0.016 ± 0.003 kHz/mmHg) in response to hindlimb contraction alone. In contracting hindlimbs, however, l-NAME significantly enhanced the vasoconstrictor responses to norepinephrine (Fig. 2 c and d).
Metabolic Modulation of α-Adrenergic Vasoconstriction Is Impaired in Contracting nNOS-Deficient mdx Mouse Hindlimb.
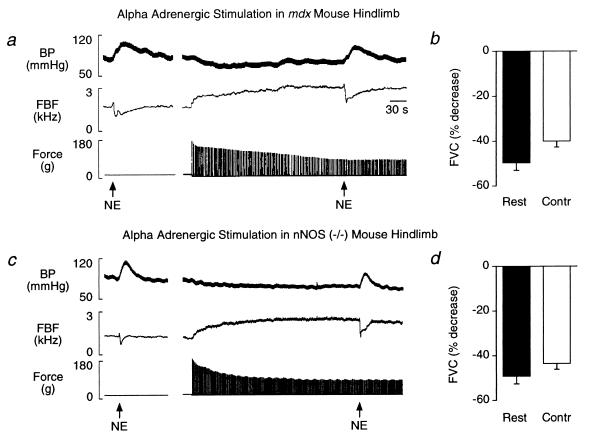
Intraarterial injection of norepinephrine into the resting hindlimb of mdx mice elicited decreases in femoral blood flow velocity (−44 ± 4%) and vascular conductance (−50 ± 3%) comparable to the vasoconstrictor responses observed in C57 mice (Fig. 3 a and b). The increases in femoral blood flow velocity and vascular conductance in response to hindlimb contraction alone in mdx mice also were similar to responses in C57 mice (Table 2). In contrast to C57 mice, in mdx mice norepinephrine-mediated vasoconstriction was not attenuated by hindlimb contraction (femoral blood flow velocity, −35 ± 3%; conductance, −40 ± 3%; Fig. 3 a and b).
Figure 3.
Metabolic inhibition of α-adrenergic vasoconstriction is defective in contracting nNOS-deficient skeletal muscle of mdx and nNOS knockout mice. (a and c) Segments of original records from experiments in an mdx mouse (a) and in an nNOS knockout mouse (c) showing the arterial blood pressure (BP) and femoral blood flow velocity (FBF) responses to intraarterial injection of norepinephrine (NE) in resting and contracting hindlimb. In both mice, NE injection in resting hindlimb increased arterial pressure and decreased femoral blood flow velocity (Left in a and c). This NE-mediated vasoconstriction was not attenuated in contracting hindlimbs, indicating that metabolic modulation of α-adrenergic vasoconstriction was impaired in nNOS-deficient muscle (Right in a and c). (b and d) Summary data showing the NE-mediated decreases in calculated femoral vascular conductance (FVC) in resting and contracting (Contr) hindlimb. The NE-mediated decreases in vascular conductance were not attenuated by hindlimb contraction in mdx mice (b; n = 10) or in nNOS knockout mice (d; n = 6).
This experiment was repeated in the mdx mice after treatment with l-NAME to determine whether there would be a further enhancement of adrenergic vasoconstriction in contracting nNOS-deficient muscle after inhibition of eNOS. In mdx mice, l-NAME did not alter norepinephrine-mediated vasoconstriction in resting hindlimb (femoral blood flow velocity, −40 ± 2%; conductance, −40 ± 2%). l-NAME did not affect the initial force produced by the contracting hindlimb (199 ± 13 g), but did decrease the rate of decline (t1/2 = 4.6 ± 0.6 min before vs. 8.0 ± 1.1 min after l-NAME treatment). l-NAME also did not affect the increases in femoral blood flow velocity (+1.03 ± 0.06 kHz) or vascular conductance (+0.015 ± 0.002 kHz/mmHg) in response to hindlimb contraction alone. Importantly, l-NAME caused no further enhancement of norepinephrine-mediated vasoconstriction in the contracting hindlimb of mdx mice (femoral blood flow velocity, −42 ± 3%; conductance, −47 ± 3%), suggesting that the abnormal vasoconstriction in contracting mdx muscle is due mainly to lack of nNOS with little additional contribution from eNOS.
Metabolic Modulation of α-Adrenergic Vasoconstriction Also Is Impaired in Contracting nNOS Knockout Mouse Hindlimb.
Intraarterial injection of norepinephrine into the resting hindlimb of nNOS knockout mice elicited decreases in femoral blood flow velocity (−46 ± 4%) and vascular conductance (−49 ± 4%) comparable to the vasoconstrictor responses observed in C57 and mdx mice (Fig. 3 c and d). In response to hindlimb contraction alone, femoral blood flow velocity and vascular conductance increased in nNOS knockout mice (Table 2). Similar to the mdx mice, norepinephrine-mediated vasoconstriction was not attenuated by hindlimb contraction in the nNOS knockout mice (femoral blood flow velocity, −37 ± 3%; conductance, −44 ± 2%) (Fig. 3 c and d). Likewise, l-NAME did not further enhance norepinephrine-mediated vasoconstriction in the contracting hindlimb of nNOS knockout mice (femoral blood flow velocity, −44 ± 7%; conductance, −58 ± 3%).
DISCUSSION
The major finding of the present study is that local metabolic modulation of α-adrenergic vasoconstriction is defective in contracting nNOS-deficient skeletal muscle of both the mdx mouse and the nNOS knockout mouse. These data provide evidence for a specific inhibitory interaction between nNOS and α-adrenergic vasoconstriction in contracting skeletal muscle, suggesting a functional role for nNOS in the regulation of skeletal-muscle blood flow during exercise.
A salient feature of this study is the suggestion that skeletal-muscle nNOS should be considered as a potential source of NO that is capable of modifying vascular tone in active skeletal muscle. Because nNOS was identified only recently in skeletal muscle (14, 15), previous studies implicating NO in the control of muscle blood flow during exercise have focused on eNOS as the source of increased NO production (25, 26). In those studies, the use of nonselective pharmacological NOS inhibitors precluded identification of specific roles for eNOS in the vasculature versus nNOS in skeletal muscle. By contrast, in this study the genetic models provided by the mdx and nNOS knockout mice suggest a pivotal role for nNOS in the local metabolic modulation of skeletal-muscle blood flow during exercise.
Although both mdx mice and patients with DMD are dystrophin mutants and have nNOS-deficient skeletal muscle (16, 17), the mice typically display a milder phenotype than the patients. Skeletal muscle of mdx mice undergoes an early period of myocyte necrosis at 2–5 weeks, followed by successful regeneration so that adult mdx mice, unlike DMD patients, demonstrate little or no muscle weakness (21). These phenotypic differences between DMD patients and mdx mice have limited the utility of the mouse model for studying the muscle pathophysiology of the disease and for testing potential therapeutic strategies. However, the mild phenotype of the mdx mouse provided a distinct advantage in this study, permitting us to identify a major impairment specifically in the contraction-induced metabolic modulation of α-adrenergic vasoconstriction in the absence of any detectable abnormalities in skeletal-muscle contractile ability, baseline hemodynamics, or the general pattern of hemodynamic responses to muscle contraction.
Dystrophin also is found normally in human and rodent vascular smooth muscle, but it is absent in the vasculature of DMD patients and mdx mice (27). Although the lack of dystrophin does not appear to alter vessel morphology (28, 29), cultured aortic smooth-muscle cells from mdx mice exhibit decreased contractile ability (30). It is unlikely that dystrophin-related changes in vascular smooth-muscle function could explain the results of our study, however, because we observed normal vasoconstrictor responses in resting muscle and enhanced, not diminished, vasoconstrictor responses during hindlimb contraction in mdx mice.
Although the present data are entirely consistent with our hypothesis that contraction-induced activation of nNOS is an important mechanism underlying metabolic modulation of α-adrenergic vasoconstriction in active skeletal muscle, alternative explanations should be considered. For example, the metabolic response to muscle contraction may be altered in mdx mice because of a transition in regenerating muscle from fast-twitch glycolytic fibers to slow-twitch oxidative fibers (31–33). In addition, the lack of dystrophin and consequent disruption of the cytoskeletal organization may negatively impact the cytosolic and mitochondrial enzymes involved in energy production (34, 35). Although we cannot completely discount these alternative explanations, the conclusion that metabolic modulation of α-adrenergic vasoconstriction in contracting skeletal muscle is tightly coupled to the production of NO is supported by the observation that the mdx phenotype was mimicked both by pharmacological NOS inhibition in the C57 mice and by nNOS gene deletion in the nNOS knockout mice.
α-Adrenergic vasoconstriction has been reported to be antagonized by NO in isolated blood vessels (5, 6), in microvascular preparations of anesthetized rats and dogs (7, 8), in the hindlimb of conscious rats (9), and in the contracting hindlimb of anesthetized rats (11). This antagonistic effect of NO is more potent for α2- rather than α1-adrenergic vasoconstriction in rat skeletal-muscle microcirculation (7) and in the canine coronary vascular bed (10). Studies from our laboratory (4) have shown that α2- but not α1-adrenergic vasoconstriction is greatly attenuated during contraction of the fast-twitch gastrocnemius muscle in rats. In normal rodent skeletal muscle, nNOS is preferentially localized to the sarcolemma of fast-twitch fibers (15), the same muscle fibers in which we preferentially demonstrate attenuated α-adrenergic vasoconstriction during muscle contraction (4). In contrast, nNOS is less abundant in oxidative slow-twitch fibers (e.g., soleus muscles) (15) in which we demonstrate little or no contraction-induced attenuation of adrenergic vasoconstriction (4). Although we previously interpreted this preferential expression of attenuated vasoconstriction in contracting glycolytic gastrocnemius versus oxidative soleus muscles to suggest an important role of H+, an alternative explanation is the preferential distribution of nNOS. The stimulus for increased NO production by nNOS in skeletal muscle in our experiments is not known, but most likely is linked to membrane depolarization and the resultant increase in intracellular Ca2+ that occurs during muscle contraction.
The precise mechanism by which NO opposes α-adrenergic vasoconstriction is not known, but it may involve cGMP-dependent attenuation of receptor-mediated Ca2+ influx in vascular smooth muscle (36), reduced Ca2+ sensitivity of the contractile apparatus (37), or enhanced cytosolic removal of Ca2+ by Ca2+–ATPase (38). NO also is reported to open both Ca2+-dependent (39) and ATP-sensitive potassium (KATP) channels (40) in vascular smooth muscle. We previously showed (41) that activation of KATP channels greatly attenuates sympathetic vasoconstriction in contracting rat hindlimb, prompting the hypothesis that NO-induced activation of KATP channels could be one mechanism by which vasoconstriction is attenuated in contracting muscle.
The modulation of α-adrenergic vasoconstriction by NO is probably one of many mechanisms producing the overall hyperemic response to skeletal muscle contraction. Metabolic, neural, humoral, and mechanical factors have all been implicated in the regulation of muscle blood flow, increasing the likelihood for redundancy among these control mechanisms. Therefore, in the mdx and nNOS knockout mice, the preserved hyperemic response to contraction of nNOS-deficient muscle is most likely the result of compensation by alternative vasodilator mechanisms.
In conclusion, we propose that contraction-induced activation of nNOS plays an important role in the regulation of skeletal muscle blood flow during exercise by opposing α-adrenergic vasoconstriction in active muscle. These findings may have potential implications not only for understanding the regulation of skeletal muscle blood flow during exercise in normal, healthy humans, but also may provide a novel vascular mechanism contributing to the pathophysiology of DMD. Although nNOS deficiency alone is not sufficient to cause muscular dystrophy (42), reduced skeletal muscle nNOS may exacerbate the abnormal function of dystrophin-deficient muscle. We speculate that in DMD patients the nNOS-deficient skeletal muscles are susceptible to recurring ischemia and hypoxia because of unopposed sympathetic vasoconstriction, which may contribute to the decreased exercise tolerance and possibly to the preferential destruction of fast-twitch muscle fibers early in the course of the disease (43).
Acknowledgments
We thank Dr. Jere H. Mitchell for his support and critical review of our work. This research was supported by grants from the American Heart Association, Texas Affiliate (96G-064 to G.D.T.), the National Institutes of Health (P01-HL-06296 to J.T.S. and R.G.V.), the Muscular Dystrophy Association (to J.T.S.), and the Michaelsen Foundation and Danish Heart Foundation (to M.S.). P.L.H. is an Established Investigator of the American Heart Association.
ABBREVIATIONS
- nNOS
neuronal NO synthase
- eNOS
endothelial NO synthase
- DMD
Duchenne muscular dystrophy
- l-NAME
N-nitro l-arginine methyl ester
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
A Commentary on this article begins on page 14592.
References
- 1.Remensnyder J P, Mitchell J H, Sarnoff S J. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- 2.Burcher E, Garlick D. J Pharmacol Exp Ther. 1973;187:78–85. [PubMed] [Google Scholar]
- 3.Anderson K M, Faber J E. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- 4.Thomas G D, Hansen J, Victor R G. Am J Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- 5.Martin W, Furchgott R F, Villani G M, Jothianidine D. J Pharmacol Exp Ther. 1986;237:529–538. [PubMed] [Google Scholar]
- 6.Topouzis S, Schott C, Stoclet J C. J Cardiovasc Pharmacol. 1991;18:670–678. doi: 10.1097/00005344-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Ohyanagi M, Nishigaki K, Faber J E. Circ Res. 1992;71:188–200. doi: 10.1161/01.res.71.1.188. [DOI] [PubMed] [Google Scholar]
- 8.Jones C J, DeFily D V, Patterson J L, Chilian W M. Circulation. 1993;87:1264–1274. doi: 10.1161/01.cir.87.4.1264. [DOI] [PubMed] [Google Scholar]
- 9.Patil R D, DiCarlo S E, Collins H L. Am J Physiol. 1993;265:H1184–H1188. doi: 10.1152/ajpheart.1993.265.4.H1184. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi Y, Duncker D J, Bache R J. Circ Res. 1997;80:196–207. doi: 10.1161/01.res.80.2.196. [DOI] [PubMed] [Google Scholar]
- 11.Thomas G D, Victor R G. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer R M J, Moncada S. Biochem Biophys Res Commun. 1989;158:348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- 13.Rubanyi G M, Romero J C, Vanhoutte P M. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 14.Nakane M, Schmidt H H H W, Pollock J S, Förstermann U, Murad F. FEBS Lett. 1993;316:175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- 15.Kobzik L, Reid M B, Bredt D S, Stamler J S. Nature (London) 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 16.Brenman J E, Chao D S, Xia H, Aldape K, Bredt D S. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 17.Chang W-J, Iannaccone S T, Lau K S, Masters B S S, McCabe T J, McMillan K, Padre R C, Spencer M J, Tidball J G, Stull J T. Proc Natl Acad Sci USA. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koenig M, Hoffman E P, Bertelson C J, Monaco A P, Feener C, Kunkel L M. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman E P, Brown R H, Jr, Kunkel L M. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 20.Bulfield G, Siller W G, Wight P A L, Moore K J. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dangain J, Vrbová G. Muscle Nerve. 1984;7:700–704. doi: 10.1002/mus.880070903. [DOI] [PubMed] [Google Scholar]
- 22.Huang P L, Dawson T M, Bredt D S, Snyder S H, Fishman M C. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 23.Haywood R J, Shaffer R A, Fastenow C, Fink G D, Brody M J. Am J Physiol. 1981;241:H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Rees D D, Schulz R, Palmer R M J. Proc Natl Acad Sci USA. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAllister R M, Hirai T, Musch T I. Med Sci Sports Exercise. 1995;27:1145–1151. [PubMed] [Google Scholar]
- 26.Green D J, O’Driscoll G, Blanksby B A, Taylor R R. Sports Med. 1996;21:119–146. doi: 10.2165/00007256-199621020-00004. [DOI] [PubMed] [Google Scholar]
- 27.Miyatake M, Miike T, Zhao J, Yoshioka K, Uchino M, Usuku G. J Neurol Sci. 1989;93:11–17. doi: 10.1016/0022-510x(89)90157-3. [DOI] [PubMed] [Google Scholar]
- 28.Koehler J. Neurology. 1977;27:861–868. doi: 10.1212/wnl.27.9.861. [DOI] [PubMed] [Google Scholar]
- 29.Boland B, Himpens B, Denef J F, Gillis J M. Muscle Nerve. 1995;18:649–657. doi: 10.1002/mus.880180612. [DOI] [PubMed] [Google Scholar]
- 30.Harricane M-C, Fabbrizio E, Lees D, Prades C, Travo P, Mornet D. Cell Biol Int. 1994;18:947–958. doi: 10.1006/cbir.1994.1015. [DOI] [PubMed] [Google Scholar]
- 31.Carnwath J W, Shotton D M. J Neurol Sci. 1987;80:39–54. doi: 10.1016/0022-510x(87)90219-x. [DOI] [PubMed] [Google Scholar]
- 32.Hayes A, Lynch G S, Williams D A. Proc R Soc London Ser B. 1993;253:19–25. doi: 10.1098/rspb.1993.0077. [DOI] [PubMed] [Google Scholar]
- 33.Pastoret C, Sebille A. Histochemistry. 1993;100:271–276. doi: 10.1007/BF00270046. [DOI] [PubMed] [Google Scholar]
- 34.Chinet A E, Even P C, Decrouy A. Experientia. 1994;50:602–605. doi: 10.1007/BF01921731. [DOI] [PubMed] [Google Scholar]
- 35.Gannoun-Zaki L, Fournier-Bidoz S, Le Cam G, Chambon C, Millasseau P, Léger J J, Dechesne C A. FEBS Lett. 1995;375:268–272. doi: 10.1016/0014-5793(95)01225-4. [DOI] [PubMed] [Google Scholar]
- 36.Rapaport R M. Circ Res. 1986;58:407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura J, van Breemen C. Biochem Biophys Res Commun. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- 38.Cornwell T L, Pryzwansky K B, Wyatt T A, Lincoln T M. Mol Pharmacol. 1991;40:923–931. [PubMed] [Google Scholar]
- 39.Bolotina V M, Najibi S, Palacino J J, Pagano P J, Cohen R A. Nature (London) 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 40.Murphy M E, Brayden J E. J Physiol. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas G D, Hansen J, Victor R G. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao D S, Gorospe R M, Brenman J E, Rafael J A, Peters M F, Froehner S C, Hoffman E P, Chamberlain J S, Bredt D S. J Exp Med. 1996;184:609–618. doi: 10.1084/jem.184.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster C, Silberstein L, Hays A P, Blau H M. Cell. 1988;52:503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]