Abstract
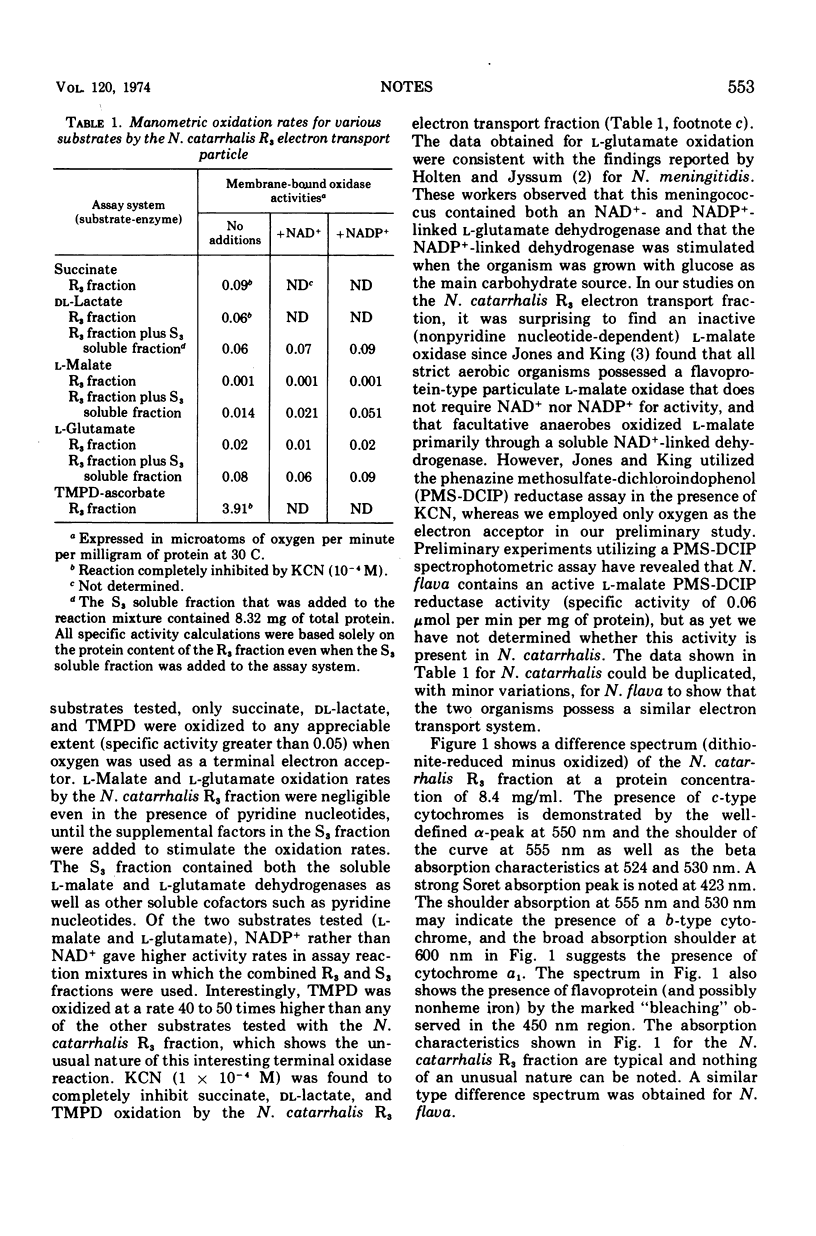
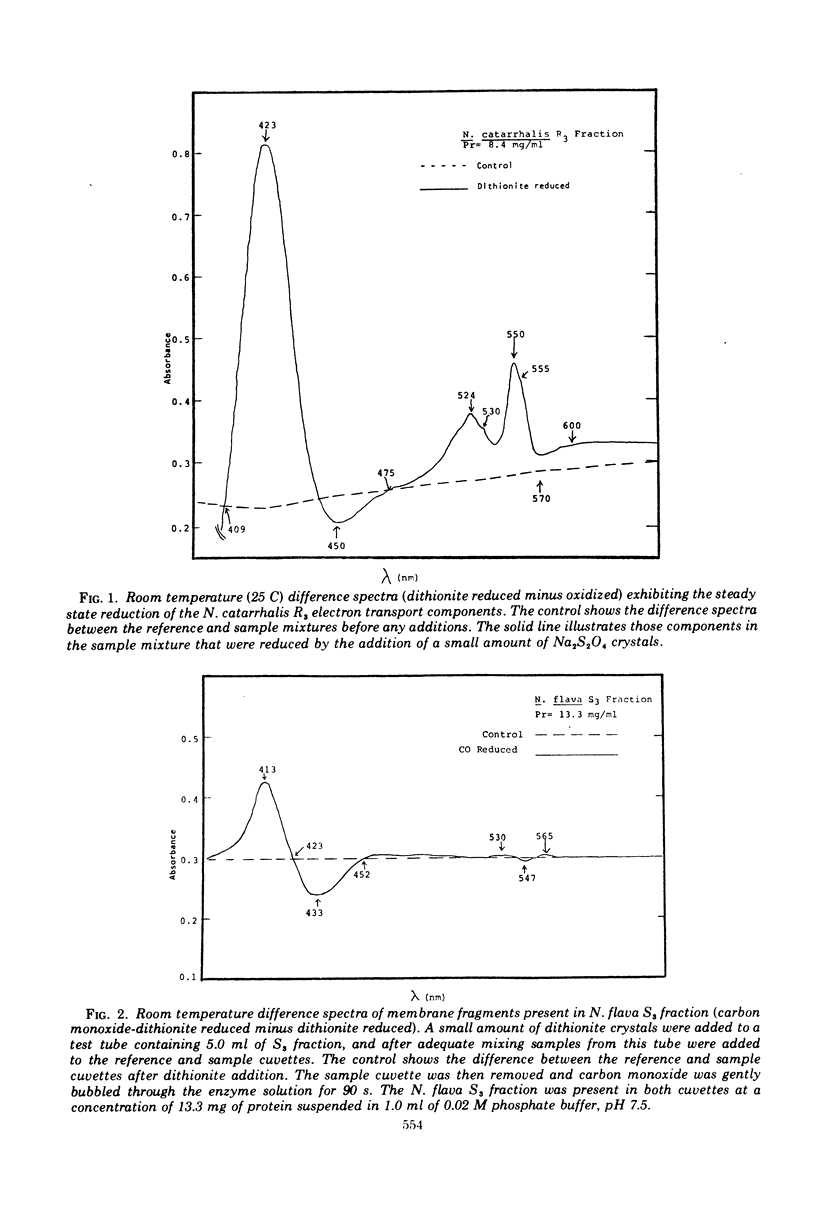
The Neisseria catarrhalis respiratory electron transport system was examined in a sonic type particulate membrane fraction and shown to have a moderately active succinate as well as nonpyridine nucleotide-dependent dl-lactate oxidoreductase and a very active tetramethyl-p-phenylenediamine oxidase. l-Malate and l-glutamate oxidation were found to be dependent on pyridine nucleotides and exclusively associated with a soluble (or nonmembranous) fraction. The primary cytochrome components in the electron transport system appear to be c-type in nature (555 nm and 550 nm) as well as cytochrome a1 (600 nm) and cytochrome o.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Holten E., Jyssum K. Glutamate dehydrogenases in Neisseria meningitidis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):43–48. doi: 10.1111/j.1699-0463.1973.tb02185.x. [DOI] [PubMed] [Google Scholar]
- JYSSUM K. Intermediate reactions of the tricarboxylic acid cycle in meningococci. Acta Pathol Microbiol Scand. 1960;48:121–132. doi: 10.1111/j.1699-0463.1960.tb04748.x. [DOI] [PubMed] [Google Scholar]
- JYSSUM K., JONER P. E. PHOSPHORYLATION COUPLED TO THE REDUCTION OF CYTOCHROME C BY HYDROXYLAMINE IN EXTRACTS FROM NEISSERIA MENINGITIDIS. Acta Pathol Microbiol Scand. 1964;62:390–398. doi: 10.1111/apm.1964.62.3.390. [DOI] [PubMed] [Google Scholar]
- Jones M., King H. K. Particulate malate oxidation in strictly aerobic bacteria: The respiratory chain of Moraxella lwoffi. FEBS Lett. 1972 May 15;22(3):277–279. doi: 10.1016/0014-5793(72)80249-7. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Aston P. R., Old L. Enzymatic oxidation of tetramethyl-p-phenylenediamine and p-phenylenediamine by the electron transport particulate fraction of Azotobacter vinelandii. J Bacteriol. 1967 Mar;93(3):1069–1078. doi: 10.1128/jb.93.3.1069-1078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Manning S., Barrera C. R. Isolation and purification of the D(-)beta-hydroxybutyric dehydrogenase of Azotobacter vinelandii. Can J Microbiol. 1968 Jul;14(7):775–783. doi: 10.1139/m68-129. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Old L. Cytochrome c oxidation by the electron transport fraction of Azotobacter vinelandii. J Bacteriol. 1968 May;95(5):1790–1797. doi: 10.1128/jb.95.5.1790-1797.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONHAZY N. E., PELCZAR M. J., Jr Oxidation of amino acids and compounds associated with the tricarboxylic acid cycle by Neisseria gonorrhoeae. J Bacteriol. 1953 Apr;65(4):368–377. doi: 10.1128/jb.65.4.368-377.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


