Abstract
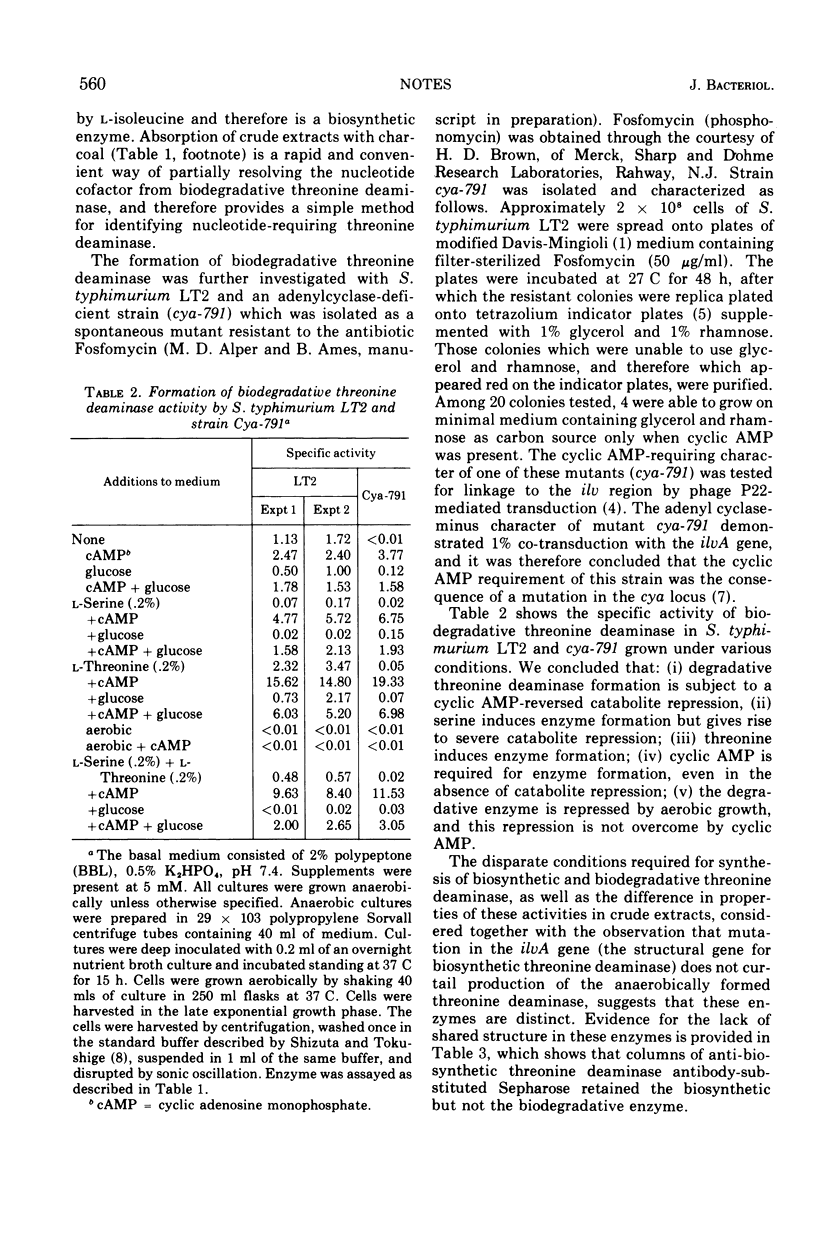
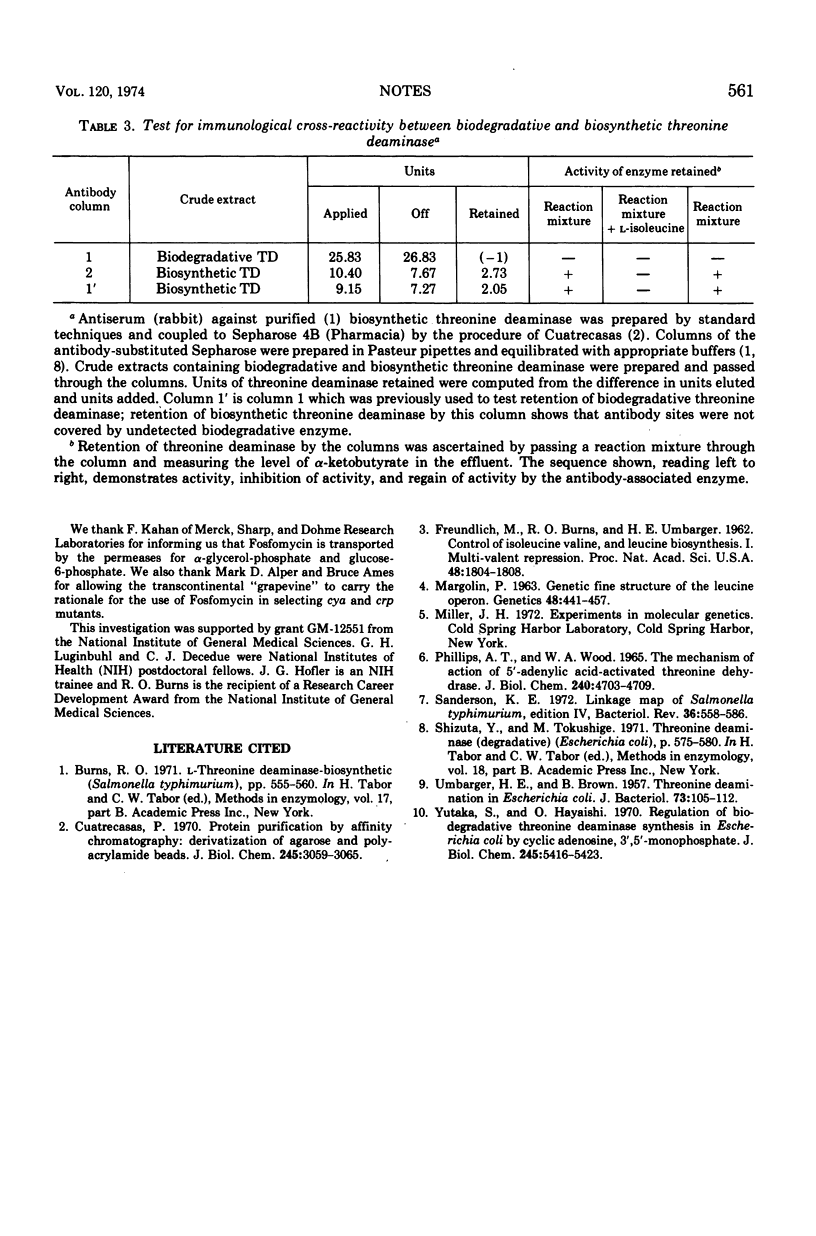
The threonine deaminase formed under anaerobic conditions by Salmonella typhimurium is induced by l-serine and l-threonine, is catabolite repressible, requires cyclic adenosine 3′,5′-monophosphate for its synthesis and adenylic acid for optimal activity, and is immunologically different from biosynthetic threonine deaminase.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. T., Wood W. A. The mechanism of action of 5'-adenylic acid-activated threonine dehydrase. J Biol Chem. 1965 Dec;240(12):4703–4709. [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuta Y., Hayaishi O. Regulation of biodegradative threonine deaminase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1970 Oct 25;245(20):5416–5423. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]



