Abstract
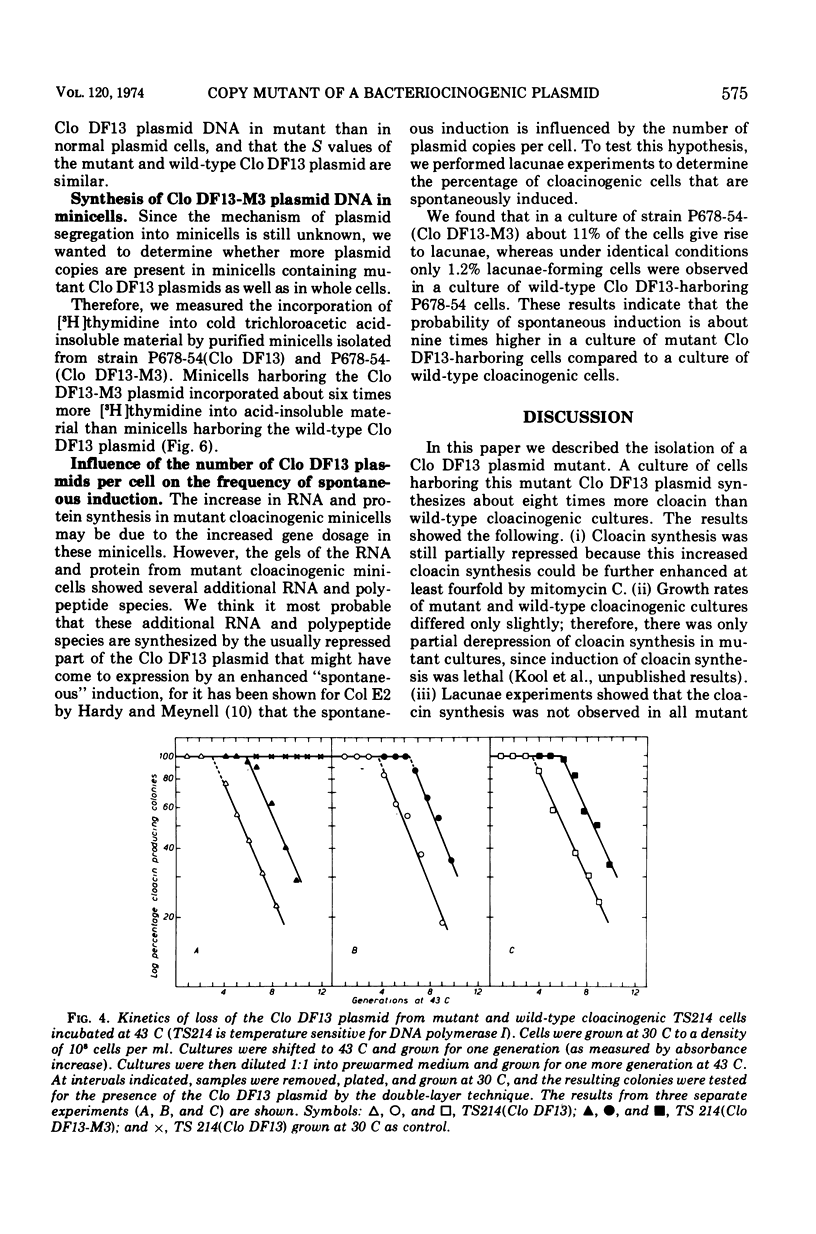
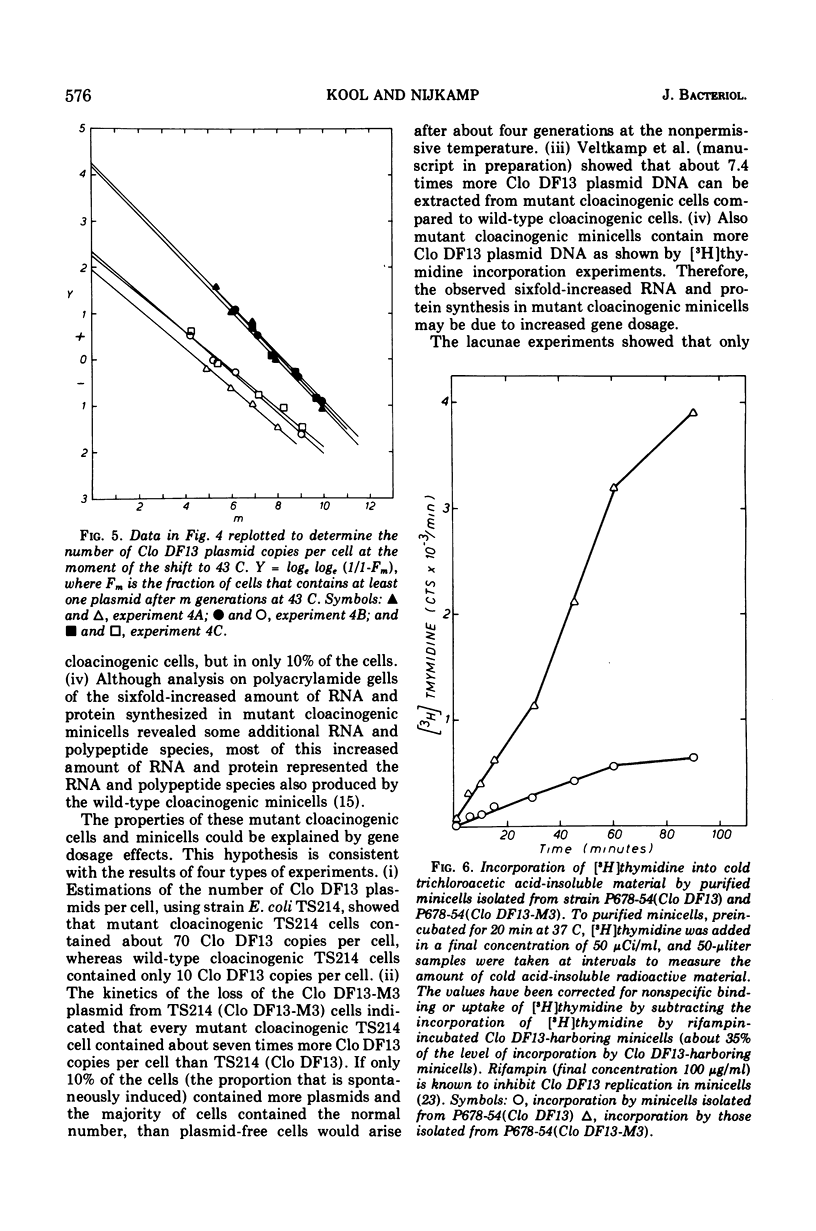
After nitrosoguanidine mutagenesis, strain Escherichia coli P678-54, bacteriocinogenic for Clo DF13, yielded a mutant strain that showed an enhanced bacteriocin production. The results from conjugation experiments indicated that the mutation, responsible for the enhanced bacteriocin production, is located on the Clo DF13 plasmid. The following properties of strains harboring the mutant Clo DF13 plasmid could be observed. (i) The bacteriocin production in these strains can be further enhanced at least fourfold by mitomycin C. (ii) The fraction of spontaneously induced cells, as revealed by lacunae experiments, in cultures of these strains is about nine times higher than in cultures of wild-type Clo DF13-harboring strains. (iii) Chromosomeless minicells from strain P678-54 harboring the mutant Clo DF13 plasmid synthesize about six times more deoxyribonucleic acid, ribonucleic acid, and protein as compared to wild-type Clo DF13-harboring minicells. (iv) Analysis of this mutant Clo DF13-specific ribonucleic acid and protein on polyacrylamide gels revealed mainly the same ribonucleic acid and polypeptide species as synthesized by the wild-type Clo DF13 minicells, but in larger amounts (Kool et al., 1974). (v) Segregation experiments, using a strain with temperature-sensitive polymerase I, show that mutant Clo DF13-harboring cells contain an average of 70 Clo DF13 copies per cell, whereas wild-type Clo DF13-harboring cells contain only about 10 Clo DF13 copies per cell. The data presented in this paper indicate that the mutation on the Clo DF13 plasmid leads to an altered control of Clo DF13 replication and results in an enhanced number of Clo DF13 copies per cell. As a secondary effect, this enhanced number of Clo DF13 copies enhances the probability of “spontaneous” induction per cell. Since the mutation is plasmid specific and affects the number of plasmid copies produced, one can conclude that the Clo DF13 plasmid is not dependent solely on chromosomal information, but that at least plasmid base sequences are involved in Clo DF13 plasmid replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Planta R. J., Stouthamer A. H. Effect of a bacteriocin produced by Enterobacter cloacae on protein biosynthesis. Biochim Biophys Acta. 1971 Jun 17;240(1):123–136. [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkacz B. W., Sherratt D. J. Segregation kinetics of colicinogenic factor col E1 from a bacterial population temperature sensitive for DNA polymerase I. Mol Gen Genet. 1973;121(1):71–75. doi: 10.1007/BF00353694. [DOI] [PubMed] [Google Scholar]
- Goebel W. The influence of DNA A and DNA C mutations on the initiation of plasmid DNA replication. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1000–1007. doi: 10.1016/0006-291x(73)90026-0. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. G., Meynell G. G. A model relating the replication and expression of colicin factor E2-P9. Genet Res. 1972 Dec;20(3):331–334. doi: 10.1017/s0016672300013847. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Nijkamp H. J. Isolation and characterization of immunity temperature sensitive mutants of Enterobacter cloacae harbouring the cloacinogenic factor DF13. Mol Gen Genet. 1972;114(4):312–324. doi: 10.1007/BF00267500. [DOI] [PubMed] [Google Scholar]
- Kool A. J., Pranger M., Nijkamp H. J. Proteins synthesized by a non-induced bacteriocinogenic factor in minicells of Escherichia coli. Mol Gen Genet. 1972;115(4):314–323. doi: 10.1007/BF00333170. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZEKI H., STOCKER B. A., DE MARGERIE H. Production of colicine by single bacteria. Nature. 1959 Aug 1;184:337–339. doi: 10.1038/184337a0. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B. H., Cavalieri L. F., Ungers G. The negative control mechanism for E. coli DNA replication. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1410–1417. doi: 10.1073/pnas.63.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Tieze G. A. Bacteriocin production by members of the genus Klebsiella. Antonie Van Leeuwenhoek. 1966;32(2):171–182. doi: 10.1007/BF02097457. [DOI] [PubMed] [Google Scholar]
- Tieze G. A., Stouthamer A. H., Jansz H. S., Zandberg J., van Bruggen E. F. A bacteriocinogenic factor of Enterobacter cloacae. Mol Gen Genet. 1969;106(1):48–65. [PubMed] [Google Scholar]
- Veltkamp E., Barendsen W., Nijkamp H. J. Influence of protein and ribonucleic acid synthesis on the replication of the bacteriocinogenic factor Clo DF13 in Escherichia coli cells and minicells. J Bacteriol. 1974 Apr;118(1):165–174. doi: 10.1128/jb.118.1.165-174.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp E., Nijkamp H. J. The role of DNA polymerase I, II and 3 in the replication of the bacteriocinogenic factor Clo DF 13. Mol Gen Genet. 1973 Sep 27;125(4):329–340. doi: 10.1007/BF00276588. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Tieze G. A., Wendelaar Bonga S., Stouthamer A. H. Purification and genetic determination of bacteriocin production in Enterobacter cloacae. J Bacteriol. 1968 Feb;95(2):631–640. doi: 10.1128/jb.95.2.631-640.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]