Abstract
A major concern in plant morphogenesis is whether cortical microtubules are responsible for the arrangement and action of β-glucan synthases in the plasma membrane. We prepared isolated plasma membrane sheets with cortical microtubules attached and tested whether β-glucan synthases penetrated through the membrane to form microfibrils and whether these synthases moved in the fluid membrane along the cortical microtubules. This technique enabled us to examine synthesis of β-glucan as a fiber with a two-dimensional structure. The synthesis of β-glucan microfibrils was directed in arrays by cortical microtubules at many loci on the membrane sheets. The microfibrils were mainly arranged along the microtubules, but the distribution of microfibrils was not always parallel to that of the microtubules. The rate of β-glucan elongation as determined directly on the exoplasmic surface was 620 nm per min. When the assembly of microtubules was disrupted by treatment with propyzamide, the β-glucans were not deposited in arrays but in masses. This finding shows that the arrayed cortical microtubules are not required for β-glucan synthesis but are required for the formation of arranged microfibrils on the membrane sheet.
Keywords: cortical microtubule/UDP-glucose/taxol
The direction of plant cell expansion, which is driven by turgor (1), is thought to be determined by the organization of the innermost cellulose microfibrils, i.e., those closest to the plasma membrane, even though multiple layers of differently directed microfibrils have been laid down earlier in the cell wall (2). Plant morphogenesis therefore also may be controlled by the orientation of newly formed cellulose microfibrils, which, in turn, may be controlled by the arrangement of cortical microtubules. This hypothesis is based on the observation that microtubule rearrangements occur just before similar reorientation of cellulose microfibrils (3, 4). There is, thus, evidence that the arrangement of microtubules is related to the orientation of newly deposited microfibrils in plants (5, 6) and that microtubule rearrangements occur in a continuous manner (7). However, nothing is known about the linkage between microtubules and microfibrils or the mechanism that controls the arrangement of cortical microtubules. In this report, we demonstrate that the “rails” of cortical microtubules orient β-glucan microfibrils that are synthesized enzymatically on isolated tobacco plasma membrane sheets.
Based on freeze-fracture electron microscopy, Giddings and Staehelin (8) suggested the most plausible mechanism for microtubule-microfibril parallelism. They proposed that cellulose synthases can move between microtubule guide rails in the plane of the plasma membrane and that the enzymes cannot cross the guide rails. Melan (9) showed that the alignment of newly deposited microfibrils closely corresponded to microtubule patterns on cell ghosts derived from pea protoplasts cultured in a medium containing taxol. Based on the cell ghost technique (10), we have prepared plasma membrane sheets in which β-glucan synthase complexes form β-glucan microfibrils along the guide rails of cortical microtubules. This report shows the parallelism between cortical microtubules and newly formed β-glucan microfibrils in a cell-free system.
MATERIALS AND METHODS
Preparation of Protoplasts.
Protoplasts were prepared from tobacco BY-2 cells that had been cultured for 4 days at the logarithmic phase of growth in a medium composed of Linsmaier-Skoog’s basal salts containing 3% sucrose and 0.2 mg/l 2,4-dichlorophenoxyacetic acid (11). The cell wall was digested by shaking the cells at 30°C for 1.5 h in a mixture of 0.1% pectin-lyase preparation (Pectolyase Y-23, Seishin, Tokyo), 1% cellulase (Cellulase Onozuka RS, Yakulto, Tokyo), and 0.45 M mannitol at pH 5.5. After the protoplasts were released from the digested cells, they were collected by centrifugation at 500 × g for 2 min and washed four times in 0.6 M mannitol. To preserve the ordered arrangement of microtubules in the protoplasts, 10 μM taxol was always added to the digestion and washing solutions during preparation of protoplasts.
Preparation of Plasma Membrane Sheets.
An aliquot of the protoplast suspension was transferred to a coverslip that had been coated with 0.5 mg/ml of poly-l-lysine. Five minutes later, the protoplasts were subjected to lysis in a low-osmotic buffer consisting of 50 mM Pipes/KOH (pH 7.0), 2 mM MgCl2, and 5 mM EGTA (12). The lysate was washed with 10 mM Hepes/KOH (pH 7.0), which left disk-shaped protoplast ghosts attached to the coverslip. To preserve the ordered arrangement of microtubules in the protoplast ghosts, 10 μM taxol was always added in the low-osmotic and washing solutions for preparation of the plasma membrane sheets.
In Situ Synthesis of β-Glucan.
The membrane sheets were incubated on a coverslip with 5 mM UDP-glucose in a buffer containing 5 mM MgCl2, 5 mM CaCl2, 5 μM taxol, and 10 mM Hepes/KOH (pH 7.0) in a total volume of 15 μl at 27°C. To obtain radioactive β-glucan, 5 mM UDP-[14C]glucose (27 mCi/mmol) was used as a substrate. β-Glucan also was synthesized in the membrane sheets with 50 mM sucrose and 5 mM UDP in the buffer.
Microscopic Observations.
The membrane sheets on coverslips were fixed with 50 mM Pipes/KOH buffer (pH 7.0), containing 3.7% formalin, 5 mM EGTA, and 2 mM MgCl2 at room temperature for 30 min. Microtubules were stained with mouse mAbs raised against chicken α-tubulin (IgG) and fluorescein isothiocyanate (FITC)-conjugated rabbit antibodies raised against mouse IgG. β-Glucan microfibrils were stained with 0.01% Calcofluor and washed three times with 10 mM PBS. Specimens then were examined under a Zeiss Axioskop microscope equipped with epifluorescence illumination using a 100× oil immersion objective lens (Zeiss) and FITC and UV filter sets (FITC-cut filter, KT 480) and were photographed with T-MAX 400 film (Kodak).
To observe the association of β-glucan microfibrils (blue) to cortical microtubules (red) on the membrane sheets, an Olympus inverted microscope equipped for epifluorescence with a 60× oil immersion objective lens (Olympus) and both 4′,6-diamidino-2-phenylindole and fluorescein isothiocyanate filter sets were used. The optical section images were collected on the charge-coupled device at a focus step of 60 optical sections for one specimen.
To determine fiber length, statistical analysis was carried out by using data from micrographs of microfibrils that had been selected randomly from fluorescent fibers. The micrographs were clear enough to allow each microfibril to be discerned. To determine the length of fibers, about 10 micrographs of β-glucan microfibrils on the membrane sheets were chosen for each analysis.
Product Analysis.
The β-glucans formed were hydrolyzed with 50 units of endo-cellulase preparation from Trichoderma sp. (Megazyme, Sydney, Australia) at 40°C for 72 hr, and the hydrolyzate was subjected to paper chromatography with 1-propanol/ethyl acetate/water (3:2:1, vol/vol) as solvent. The area corresponding to cellobiose on the paper was excised, and its radioactivity counted in 5 ml of a toluene-based scintillator. The recovery of cellobiose per total radiactivity incorporated is shown. Methylation of β-glucan products was performed by the method of Harris et al. (13). The methylated derivatives were separated by TLC on silica gel at 30°C for 120 min by using 1-hexane/diethyl ether/acetic acid (6:3:1, vol/vol) as solvent (14). T-Glc, 3-Glc, and 4-Glc indicate the derivatives obtained from terminal, 3-linked and 4-linked glucosyl residues, respectively.
RESULTS
Synthesis of β-Glucan on Protoplasts.
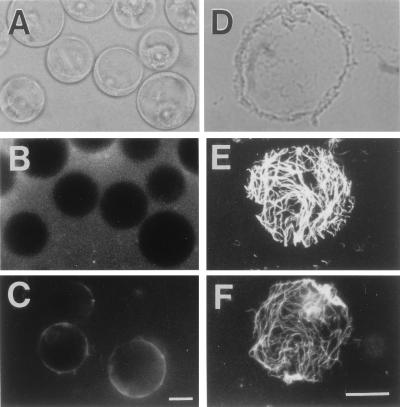
The protoplasts prepared from tobacco cells were suspended in the culture medium composed of Linsmaier-Skoog’s basal salts containing 3% sucrose, 0.2 mg/l 2,4-dichlorophenoxyacetic acid, 10 μM taxol, and 0.5 M mannitol. After incubation at 27°C for 30 min, the protoplasts produced a small amount of β-glucans on their surface (Fig. 1 A–C). After a 30-min culture, the protoplasts were transferred to a coverslip coated with polylysine. The distribution of their intercellular organelles remained normal (12). They then were subjected to lysis with a low osmotic buffer to leave disk-shaped protoplast ghosts attached on the coverslip (Fig. 1D), which is called plasma membrane sheet here. The double staining using immunofluorescence for microtubules and Calcofluor for β-glucans in the presence of taxol showed that the plasma membrane sheet had orderly arranged cortical microtubules on the (upper) surface (Fig. 1E) and β-glucan microfibrils between the membrane sheet and polylysine-coated coverslip. β-Glucan microfibrils were observed to be arranged along the microtubules (Fig. 1F). This finding is in full agreement with earlier observations that microtubules and microfibrils were arranged close to each other. It should be noted, however, that the pattern of microfibrils was similar but not identical to that of the microtubules.
Figure 1.
Formation of nascent β-glucan fibrils on protoplasts. (A) Protoplasts of tobacco cells. (B) Freshly prepared protoplasts at the onset of incubation in 0.01% Calcofluor solution. (C) Calcofluor staining of nascent β-glucan regenerated on protoplasts after 30 min of incubation. (D) A phase-contrast image of the plasma membrane sheet. (E) An immunofluorescence micrograph of cortical microtubules on the membrane sheet. (F) Calcofluor staining of the microfibrils of nascent β-glucans. (Magnification bar is 12 μm.)
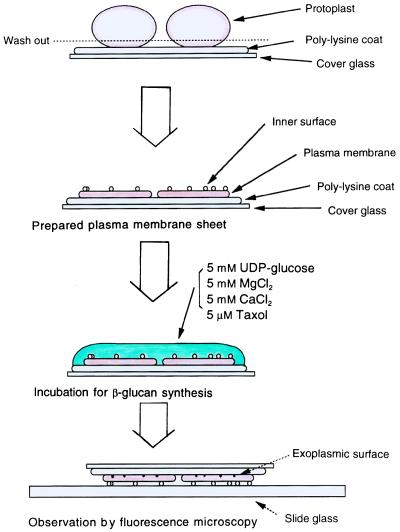
Synthesis of β-Glucan Microfibrils on Plasma Membrane Sheets.
The in situ synthesis of β-glucan microfibrils on an isolated plasma membrane sheet is summarized in Fig. 2. This technique enables us to measure synthesis of β-glucan as a fiber with a two-dimensional structure. Freshly prepared protoplasts without any β-glucans on their surfaces (Fig. 1 A and B) were immediately transferred onto a coverslip and subjected to lysis. Taxol (10 μM) was always present in the digestion and washing solutions during preparation of protoplasts and in the low osmotic washing solutions for preparation of plasma membrane sheets. The membrane sheets then were incubated with 10 mM Hepes/KOH (pH 7.0) containing 5 mM UDP-glucose, 5 mM MgCl2, 5 mM CaCl2, and 5 μM taxol. In the presence of UDP-glucose, β-glucan microfibrils were formed at the interface between the membrane sheet and the polylysine-coated coverslip.
Figure 2.
Procedure for the preparation of plasma membrane sheets and the synthesis of β-glucan microfibrils. Freshly prepared protoplasts without any β-glucans on their surfaces (Fig. 1 A and B) were immediately transferred to a coverslip and subjected to lysis. The membrane sheets thus prepared then were incubated with 5 mM UDP-glucose containing 5 μM taxol, 5 mM MgCl2, 5 mM CaCl2 and 10 mM Hepes/KOH (pH 7.0) in a total volume of 15 μl on a coverslip.
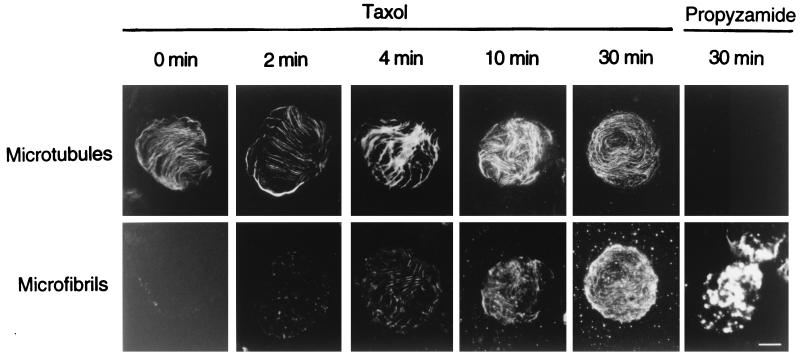
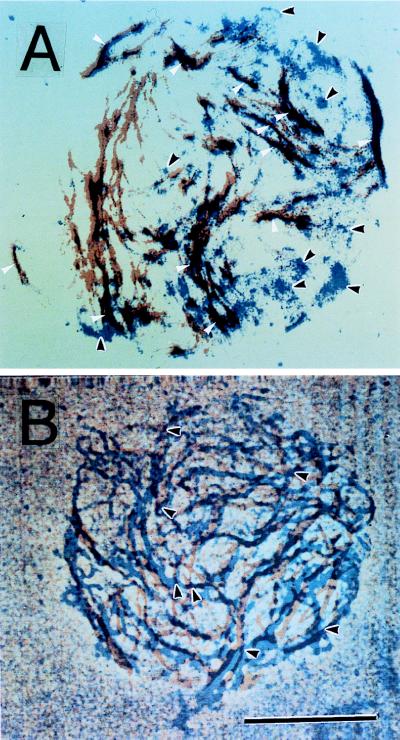
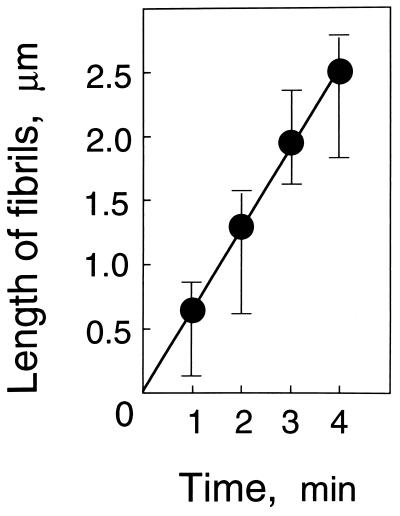
The microfibrils appeared to be formed as short fibers at many loci within a few minutes after the start of incubation, and longer fibers were synthesized during incubation for 10 min (Fig. 3). The microfibrils formed during incubation were arranged in close parallel to the microtubules. However, when the ordered structure of microtubules was disrupted by treatment with propyzamide during the preparation of protoplasts, β-glucans were deposited in masses on the membrane sheet and not in arrays (Fig. 3). Therefore, arrayed cortical microtubules are required for the formation of arranged microfibrils on the membrane sheet. The formation of microfibrils was visualized when UDP-glucose was used at a concentration of >5 mM. This result probably is caused by the level of fluorescence intensity as a function of the amount of β-glucan present because high concentrations of UDP-glucose cause an increase in β-glucan products. When the arrays of β-glucan microfibils were compared with those of cortical microtubules by using a charge-coupled device for optical sectioning microscopy, the microfibrils were found to be largely arranged along the microtubules, but the distribution of microfibrils did not always follow that of the microtubules (Fig. 4). Some strands or masses of microfibrils appeared to form where the fluorescence of cortical microtubules was invisible (Fig. 4A), and a few microfibrils seemed to be crossing the microtubules (Fig. 4B). The length of β-glucan microfibrils was directly measured 1, 2, 3, and 4 min after the start of incubation, and the rate of β-glucan elongation on the membrane sheets was calculated to be about 620 nm per min (Fig. 5). Ten micrographs taken at each time point were used for measurement and calculation.
Figure 3.
Synthesis of β-glucan microfibrils from UDP-glucose on plasma membrane sheets. Either 10 μM taxol or 100 μM propyzamide was always present in the digestion, washing, and culture solutions during preparation of protoplasts and in the low osmotic washing solutions for preparation of the membrane sheets. (Magnification bar is 8 μm.)
Figure 4.
Superimposition of images of β-glucan microfibrils and cortical microtubules. (A) Deposition difference between microfibrils and microtubules. White arrows indicate the congruence of microfibrils and microtubules, and black arrows indicate cellulose microfibrils or masses deposited independent from the microtubules. (B) Crossing of microfibrils over microtubules. Black arrows indicate a microfibril crossing over a microtubule. Microfibrils are shown in blue and microtubules in red, and both colors were combined in the combined regions (purple). (Magnification bar is 5 μm.)
Figure 5.
Length of β-glucan microfibrils formed on plasma membrane sheets during incubation with UDP-glucose.
When sucrose and UDP instead of UDP-glucose were present on the membrane sheet, β-glucan microfibrils still appeared to be formed during incubation (Fig. 6). This finding suggests that sucrose synthase is present on the plasma membrane sheet as suggested by Amor et al. (15). We have not detected the presence of sucrose synthase by using an antibody against mung bean sucrose synthase (16), probably because the antibody only weakly recognizes tobacco sucrose synthase.
Figure 6.
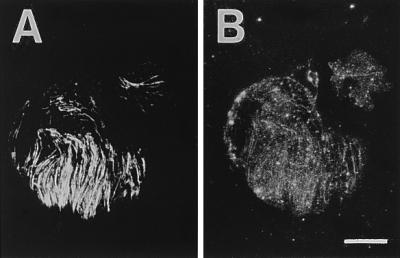
Synthesis of β-glucan microfibrils from sucrose plus UDP on isolated plasma membrane sheets. (A) Immunofluorescent microtubules. (B) Calcofluor staining of microfibrils. (Magnification bar is 8 μm.)
Analysis of β-Glucan Microfibrils.
Because similar amounts of labeled glucose were incorporated from UDP-[14C]glucose into microfibrils both in the presence and absence of microtubules (Table 1), we concluded that the presence of cortical microtubules did not affect the incorporation of glucose into β-glucan on the plasma membrane sheets. Although some 1,4-β-glucosyl linkages were detected in the presence of cortical microtubules, 1,3-β-glucan was formed from UDP-glucose on the plasma membrane sheets. This finding is in agreement with earlier observations (17–21) that many plasma membrane preparations produce mainly 1,3-β-glucan with a very small amount of 1,4-β-glucan.
Table 1.
Product analysis of β-glucan formed from UDP-glucose on plasma membrane sheets
| Additive | Total incorporation, cpm | Enzyme digestion (cellobiose), % | Methylation analysis, %
|
||
|---|---|---|---|---|---|
| T-Glc | 3-Glc | 4-Glc | |||
| None | 51,000 | 1 | 5 | 93 | 2 |
| Propyzamide | 48,500 | 1 | 5 | 94 | 1 |
| Taxol | 47,000 | 5 | 8 | 83 | 9 |
T-Glc, 3-Glc and 4-Glc indicate the derivatives obtained from terminal, 3-linked, and 4-linked glucosyl residues, respectively.
It is well known that both 1,3-β-glucan and 1,4-β-glucan are deposited on the surface of the protoplasts at the early stage of wall regeneration (22, 23). The relative amount of 1,4-β-glucan in all β-glucans is about 20–80% depending on the intactness of protoplasts. However, it is uncertain whether or not the fibers contain a higher level of 1,4-β-glucan in addition to 1,3-β-glucan (Fig. 1F).
DISCUSSION
We have demonstrated that β-glucan microfibrils formed during incubation of plasma membrane sheets are arranged in close parallel to the cortical microtubules that are present on the inner surface of the membrane. The β-glucan synthases penetrate the isolated plasma membrane and deposit the microfibrils on the outer face of the membrane (Fig. 3). This phenomenon indicates that the β-glucan synthases or β-glucan primers (24) move in the membrane sheet along the cortical microtubules to deposit microfibrils in arrays. The synthesis of β-glucan microfibrils was directed in arrays by cortical microtubules at many loci on the isolated membrane sheets. Each microfibril appeared to be thinner than each microtubule, and there were strong and weak regions of fluorescence in each microfibril. It is uncertain whether each microfibril is composed of short fibers connected to each other or whether they constitute an assembly of several elementary fibrils, being formed by several β-glucan synthase complexes. Thus, it is also uncertain whether multiple β-glucan conjugates move together to form grouped microfibrils in a larger array or whether a single conjugate forms a single elementary fibril that associates with other fibrils to form a larger structure. This idea may be the reason the length of each microfibril could be measured within 4 min after the start of incubation. The rate of microfibril elongation calculated as 620 nm/min (Fig. 5) was quite lower than that of 931 nm/min in maize and that of 1,283 nm/min in cress (25). The active β-glucan synthases probably are localized in the membrane sheets, although the distribution of β-glucan microfibrils did not always follow that of microtubules (Fig. 4A). Because some microfibrils appeared to form where the fluorescence of microtubules was not visible (Fig. 4B), there might have been a single cortical microtubule that was hard to detect.
β-Glucans were deposited in masses on the plasma membrane sheets, when the ordered structure of microtubules was disrupted by treatment with propyzamide during the preparation of protoplasts (Fig. 3). Elimination of cortical microtubules from the isolated membrane sheet by treatment with a cold solution of KCl (26) also resulted in the formation of masses of fluorescent β-glucan (data not shown). The presence of cortical microtubules, however, did not affect the incorporation of glucose into β-glucan on the plasma membrane sheet (Table 1). This finding indicates that the cortical microtubules are not required for β-glucan synthesis but are required for the formation of arranged microfibrils on the membrane sheets. Our findings strongly indicate that the direction of callose synthesis also is guided by the microtubules because the β-glucans formed on the plasma membrane sheets are composed mainly of 1,3-β-glucan originating from UDP-glucose (Table 1). Callose synthase has a complex interaction with UDP-glucose and various effectors, in which Mg2+ enhances the affinity for Ca2+ and causes a high degree of microfibril aggregation (20). In the case of cotton fibers, the size of callose formed from UDP-glucose with membrane preparations was measured by gel filtration. The average molecular mass was 100 kDa with a wide spread ranging from 40 to 2,000 kDa, which corresponded to an average length of 310 nm in a range of 120 to 6,100 nm. This finding may result in the strong and weak regions of fluorescence along one microfibril on the membrane sheet (Fig. 3). It also should be noted that mung bean 1,3-β-glucan synthase has been visualized as a doughnut-shaped structure, 20–30 nm in diameter, which is quite similar in size to the rosettes and terminal globules of cellulose synthase (20).
β-Glucan synthase may bind to microtubules or microtubule-associated proteins directly or indirectly via other components of the enzyme complex. This suggestion is based on the fact that the gene products of celA1 (27) and pcsA2 (28), catalytic subunits of cellulose 4-β-glucosyltransferase of cotton fibers, contain Zn-binding domains (29) that may be involved in protein–protein interactions (30). The Zn-binding domain of the pcsA2 gene product is located at Cys28 to Cys83 in the N-terminal region, and the transmembrane site of the N terminus begins from Glu207 (28). Thus, the distance between the Zn-binding domain and transmembrane site is composed of an α-helix-rich 124-aa sequence that can elongate to 18.6 nm, which means that the cellulose synthase itself extends 18.6 nm to reach the cortical microtubules. This result is in agreement with an earlier finding (31–33) that, on the transverse section of plasma membrane-microtubule configurations, most cortical microtubules are spaced about 10 nm away from the membrane surface and are present in pairs or in bundles of up to six microtubules, each with cross bridges to the membrane. In some photos, several microtubules were visualized more than 10 nm away from the surface, which may be why some β-glucan synthases appeared to cross over the microtubules.
β-Glucan synthases move on the isolated membrane sheets, with the polylysine-coated coverslip serving as firm surface for the movement of the conjugates guided by the microtubules. Most of the cortical microtubules were not removed by vigorous treatment with a 1% solution of the detergent Triton X-100, which extracted the lipids from the plasma membrane. This finding indicates that the microtubules are not bound to the polylysine-coated coverslip with lipid layers but with protein-cross bridges called either microtubule-membane linkers (8) or microtubule-associated proteins (34). A cold solution of KCl probably removes the whole bridge or components of the bridges because KCl-treated isolated membrane sheets did not form any arrays of microfibrils.
Acknowledgments
We thank T. Asada for performing optical sectioning microscopy and Y. Hiraoka for advice with microscopic observation. This work was supported by a Grant-in-Aid from the Ministry of Education, Science and Culture, Japan.
References
- 1.Green P B. Annu Rev Plant Physiol. 1980;31:51–82. [Google Scholar]
- 2.Taiz L. Annu Rev Plant Physiol. 1984;35:585–657. doi: 10.1146/annurev.pp.35.060184.001255. [DOI] [PubMed] [Google Scholar]
- 3.Lang J M, Eisinger W R, Green P B. Protoplasma. 1982;110:5–14. [Google Scholar]
- 4.Roberts I N, Lloyd C W, Roberts K. Planta. 1985;164:439–447. doi: 10.1007/BF00395959. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd C W. Int Rev Cytol. 1984;86:1–51. [Google Scholar]
- 6.Giddings T H, Jr, Staehelin L A. In: The Cytoskeletal Basis of Plant Growth and Form. Lloyd C W, editor. London: Academic; 1991. pp. 85–99. [Google Scholar]
- 7.Yuan M, Shaw P J, Warn R W, Lloyd C W. Proc Natl Acad Sci USA. 1994;91:6050–6053. doi: 10.1073/pnas.91.13.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giddings T H, Jr, Staehelin L A. Planta. 1988;173:22–30. doi: 10.1007/BF00394482. [DOI] [PubMed] [Google Scholar]
- 9.Melan M A. Protoplasma. 1990;153:169–177. [Google Scholar]
- 10.Van der Vark P, Rennie P J, Connolly J A, Fowke L C. Protoplasma. 1994;105:27–43. [Google Scholar]
- 11.Nagata T, Okada K, Takebe I, Matsui C. Mol Gen Genet. 1981;184:161–165. [Google Scholar]
- 12.Sonobe S. Plant Cell Physiol. 1990;31:1147–1153. [Google Scholar]
- 13.Harris P J, Henry R J, Blakeney A B, Stone B A. Carbohydr Res. 1984;127:59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- 14.Delmer D P, Cooper G, Alexander D, Cooper J, Hayashi T, Nitsche C, Thelen M. J Cell Sci. 1985;2:33–50. doi: 10.1242/jcs.1985.supplement_2.3. [DOI] [PubMed] [Google Scholar]
- 15.Amor Y, Haigler C H, Johnson S, Wainscott M, Delmer D P. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakai T, Tonouchi N, Tsuchida Y, Mori T, Sakai F, Hayashi T. Biosci Biotech Biochem. 1997;61:1500–1503. doi: 10.1271/bbb.61.1500. [DOI] [PubMed] [Google Scholar]
- 17.Raymond Y, Fincher G B, Maclachlan G A. Plant Physiol. 1978;61:938–942. doi: 10.1104/pp.61.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpita N C, Delmer D P. Plant Physiol. 1980;66:911–916. doi: 10.1104/pp.66.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polonenko D R, Maclachlan G A. J Exp Bot. 1984;35:1342–1349. [Google Scholar]
- 20.Hayashi T, Read S M, Bussell J, Thelen M, Lin F C, Brown R M, Jr, Delmer D P. Plant Physiol. 1987;83:1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuda K, Li L, Kudlicka K, Kuga S, Brown R M., Jr Plant Physiol. 1993;101:1131–1142. doi: 10.1104/pp.101.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein A S, Montezinos D, Delmer D P. Planta. 1981;152:105–114. doi: 10.1007/BF00391181. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Polonenko D R, Camirand A, Maclachlan G. Plant Physiol. 1986;82:301–306. doi: 10.1104/pp.82.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhugga K S, Tiwari S C, Ray P M. Proc Natl Acad Sci USA. 1997;94:7679–7684. doi: 10.1073/pnas.94.14.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider B, Herth W. Protoplasma. 1986;131:142–152. [Google Scholar]
- 26.Sonobe S, Takahashi S. Plant Cell Physiol. 1994;35:451–460. [Google Scholar]
- 27.Pear J R, Kawagoe Y, Schreckengost W E, Delmer D P, Stalker D M. Proc Natl Acad Sci USA. 1996;93:12637–12642. doi: 10.1073/pnas.93.22.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihara Y, Sakai F, Hayashi T. Wood Res. 1997;84:1–6. [Google Scholar]
- 29.Kawagoe, Y. & Delmer, D. P. (1997) Plant Physiol. 114, Suppl., 85 (Abstr.).
- 30.Kuroda S, Tokunaga C, Kiyohara Y, Higuchi O, Konishi H, Mizuno K, Gill G N, Kikkawa U. J Biol Chem. 1996;271:31029–31032. doi: 10.1074/jbc.271.49.31029. [DOI] [PubMed] [Google Scholar]
- 31.Hardham A R, Gunning B E S. J Cell Biol. 1978;77:14–34. doi: 10.1083/jcb.77.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seagull R W, Heath I B. Protoplasma. 1980;103:205–229. [Google Scholar]
- 33.Lancelle S A, Callaham D A, Hepler P K. Protoplasma. 1986;131:153–165. [Google Scholar]
- 34.Jiang C J, Sonobe S. J Cell Sci. 1993;105:891–901. doi: 10.1242/jcs.105.4.891. [DOI] [PubMed] [Google Scholar]