Abstract
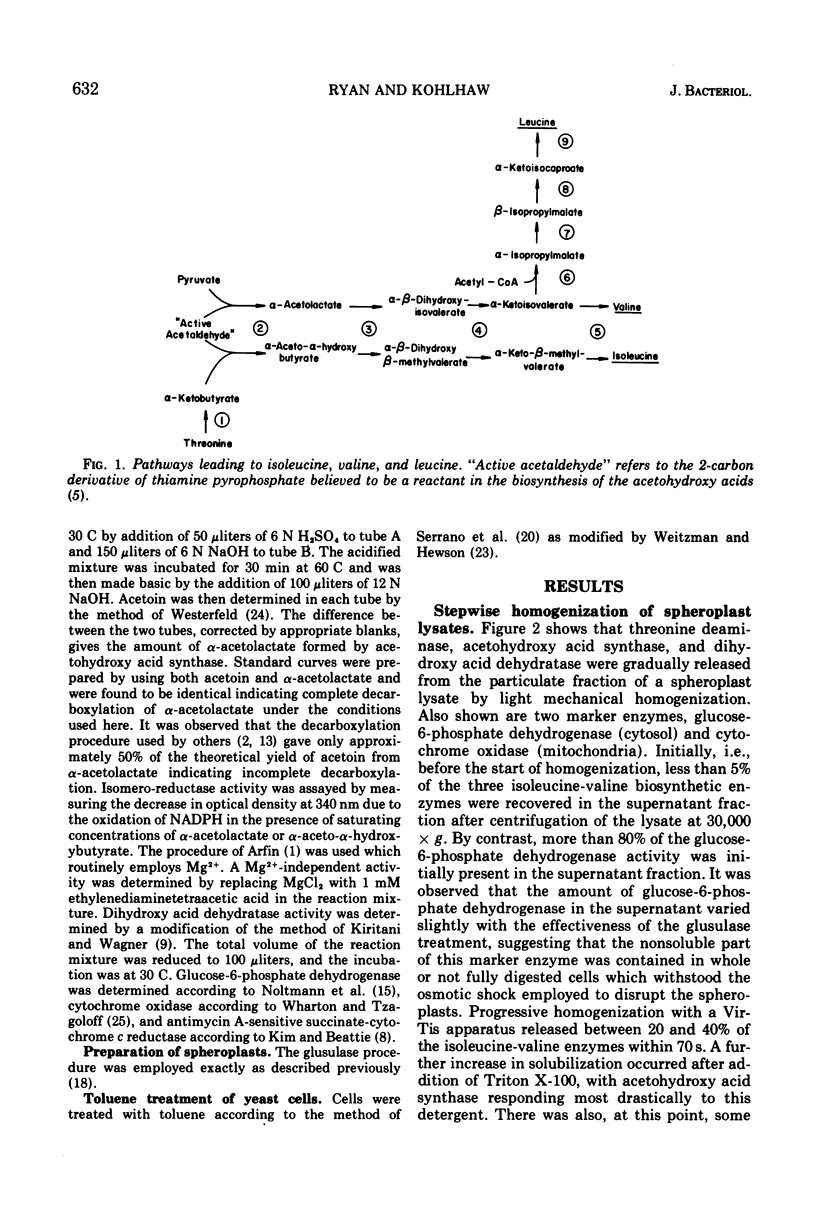
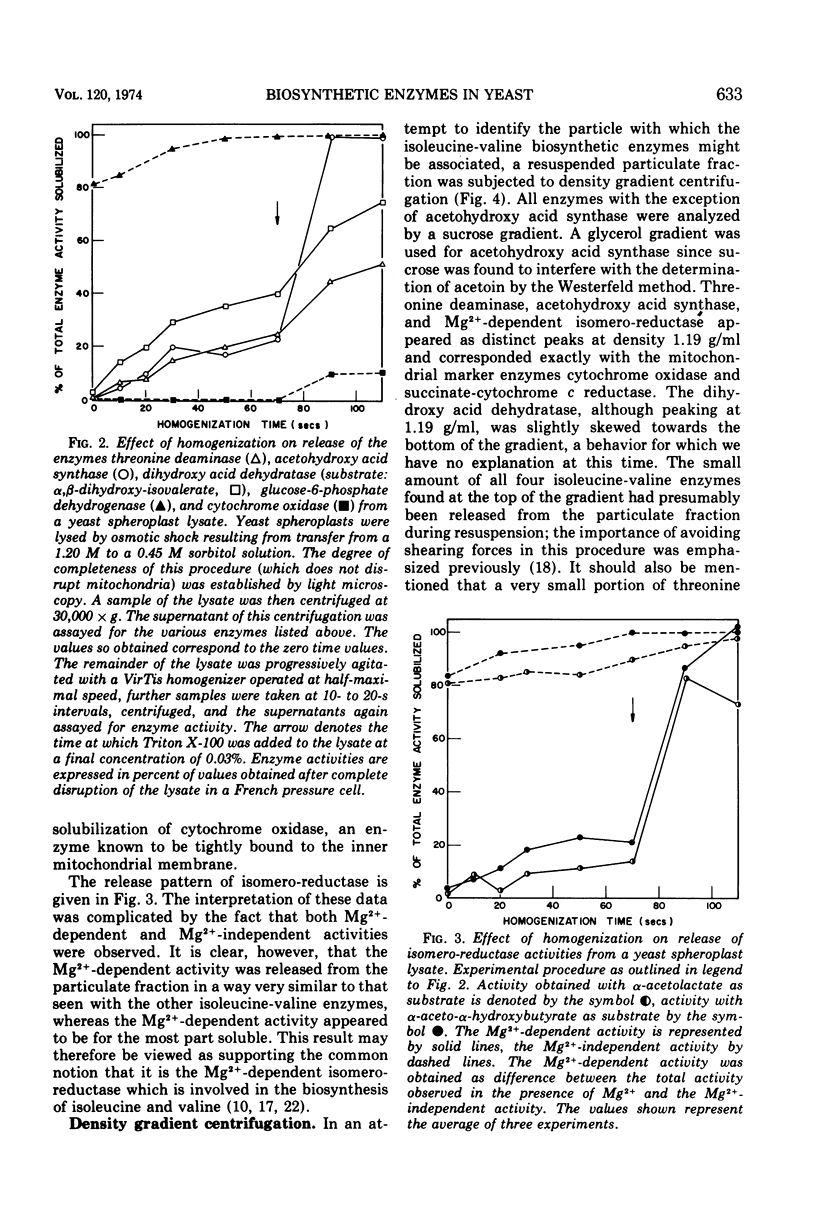
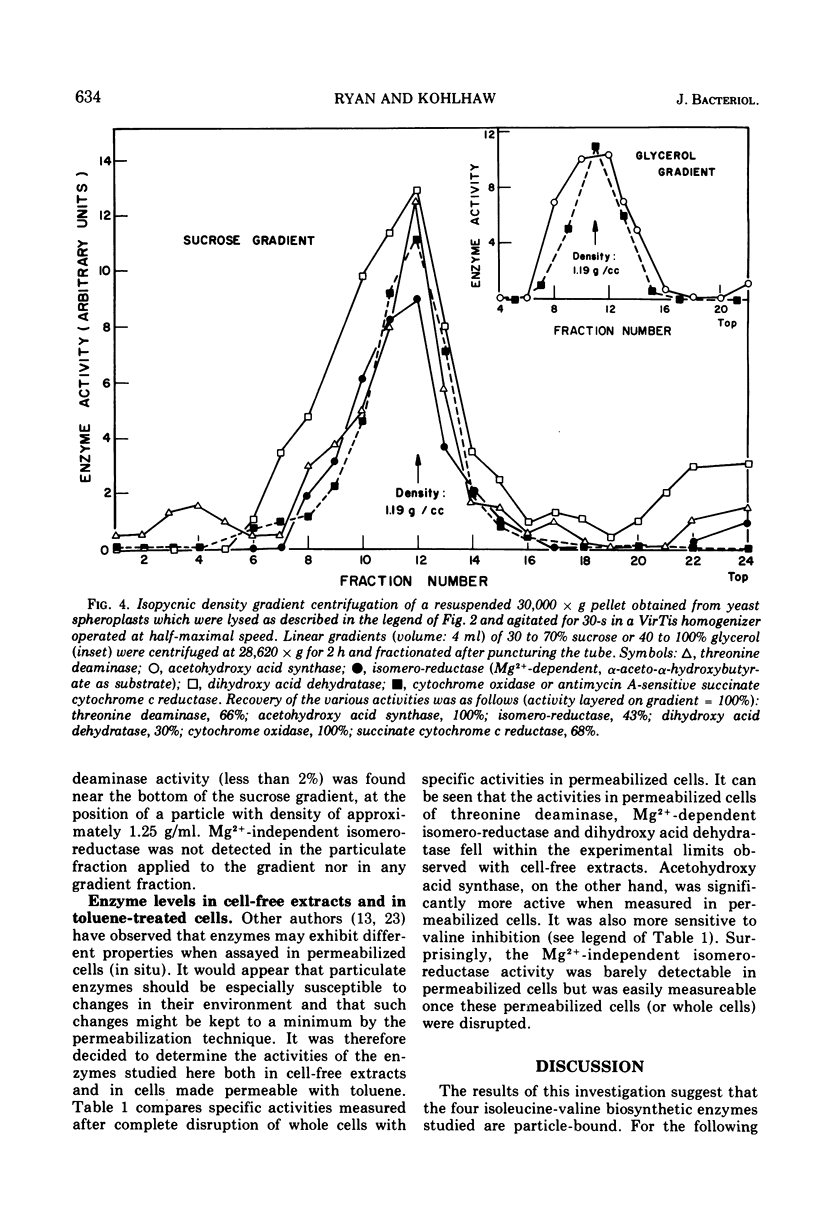
By using the method of stepwise homogenization of yeast spheroplast lysates employed previously with the leucine biosynthetic enzymes, it is shown that threonine deaminase, acetohydroxy acid synthase, Mg2+-dependent isomero-reductase, and dihydroxy acid dehydratase are particulate. Density gradient centrifugation and the behavior of marker enzymes suggest that all of the above enzymes of the isoleucine-valine biosynthetic pathway are associated with the mitochondria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUERLE R. H., FRUENDLICH M., STORMER F. C., UMBARGER H. E. CONTROL OF ISOLEUCINE, VALINE AND LEUCINE BIOSYNTHESIS. II. ENDPRODUCT INHIBITION BY VALINE OF ACETOHYDROXY ACID SYNTHETASE IN SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 Oct 23;92:142–149. [PubMed] [Google Scholar]
- Cassady W. E., Leiter E. H., Bergquist A., Wagner R. P. Separation of mitochondrial membranes of Neurospora crassa. II. Submitochondrial localization of the isoleucine-valine biosynthetic pathway. J Cell Biol. 1972 Apr;53(1):66–72. doi: 10.1083/jcb.53.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Umbarger H. E. Threonine deaminase from Bacillus subtilis. I. Purification of the enzyme. J Biol Chem. 1970 Apr 10;245(7):1736–1741. [PubMed] [Google Scholar]
- Kellems R. E., Allison V. F., Butow R. A. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. II. Evidence for the association of cytoplasmic ribosomes with the outer mitochondrial membrane in situ. J Biol Chem. 1974 May 25;249(10):3297–3303. [PubMed] [Google Scholar]
- Kellems R. E., Butow R. A. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. 3. Changes in the amount of bound ribosomes in response to changes in metabolic state. J Biol Chem. 1974 May 25;249(10):3304–3310. [PubMed] [Google Scholar]
- Kim I. C., Beattie D. S. Formation of the yeast mitochondrial membrane. 1. Effects of inhibitors of protein synthesis on the kinetics of enzyme appearance during glucose derepression. Eur J Biochem. 1973 Jul 16;36(2):509–518. doi: 10.1111/j.1432-1033.1973.tb02937.x. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Coleman W. G., Jr, Umbarger H. E. Regulation of isoleucine-valine biosynthesis in an ilvDAC deletion strain of Escherichia coli K-12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1144–1151. doi: 10.1016/0006-291x(74)90816-x. [DOI] [PubMed] [Google Scholar]
- Kuwana H., Caroline D. F., Harding R. W., Wagner R. P. An acetohydroxy acid synthetase from Neurospora crassa. Arch Biochem Biophys. 1968 Oct;128(1):184–193. doi: 10.1016/0003-9861(68)90021-0. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 2. Identification and characterization of mutants lacking the acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):502–506. doi: 10.1111/j.1432-1033.1967.tb19559.x. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- Marco R., Pestaña A., Sebastian J., Sols A. Oxaloacetate metabolic crossroads in liver. Enzyme compartmentation and regulation of gluconeogenesis. Mol Cell Biochem. 1974 Mar 8;3(1):53–70. doi: 10.1007/BF01660077. [DOI] [PubMed] [Google Scholar]
- NOLTMANN E. A., GUBLER C. J., KUBY S. A. Glucose 6-phosphate dehydrogenase (Zwischenferment). I. Isolation of the crystalline enzyme from yeast. J Biol Chem. 1961 May;236:1225–1230. [PubMed] [Google Scholar]
- Olshan A. R., Gross S. R. Role of the leu-3 cistron in the regulation of the synthesis of isoleucine and valine biosynthetic enzymes of Neurospora. J Bacteriol. 1974 May;118(2):374–384. doi: 10.1128/jb.118.2.374-384.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., WAGNER R. P., SNELL E. E. Biosynthesis of valine and i43soleucine, 3. alpha-Keto-beta-hydroxy acid reductase and alpha-hydroxy-beta-Keto acid reductoisomerase. J Biol Chem. 1960 Aug;235:2322–2331. [PubMed] [Google Scholar]
- Ryan E. D., Tracy J. W., Kohlhaw G. B. Subcellular localization of the leucine biosynthetic enzymes in yeast. J Bacteriol. 1973 Oct;116(1):222–225. doi: 10.1128/jb.116.1.222-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Gancedo J. M., Gancedo C. Assay of yeast enzymes in situ. A potential tool in regulation studies. Eur J Biochem. 1973 May 2;34(3):479–482. doi: 10.1111/j.1432-1033.1973.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Takenaka S., Kuwana H. Control of acetohydroxy acid synthetase in Neurospora crassa. J Biochem. 1972 Nov;72(5):1139–1145. doi: 10.1093/oxfordjournals.jbchem.a130001. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B., EYRING E. J. Isoleucine and valine metabolism in Escherichia coli. IX. Utilization of acetolactate and acetohydroxybutyrate. J Biol Chem. 1960 May;235:1425–1432. [PubMed] [Google Scholar]
- Weitzman P. D., Hewson J. K. In situ regulation of yeast citrate synthase. Absence of ATP inhibition observed in vitro. FEBS Lett. 1973 Oct 15;36(2):227–231. doi: 10.1016/0014-5793(73)80374-6. [DOI] [PubMed] [Google Scholar]


