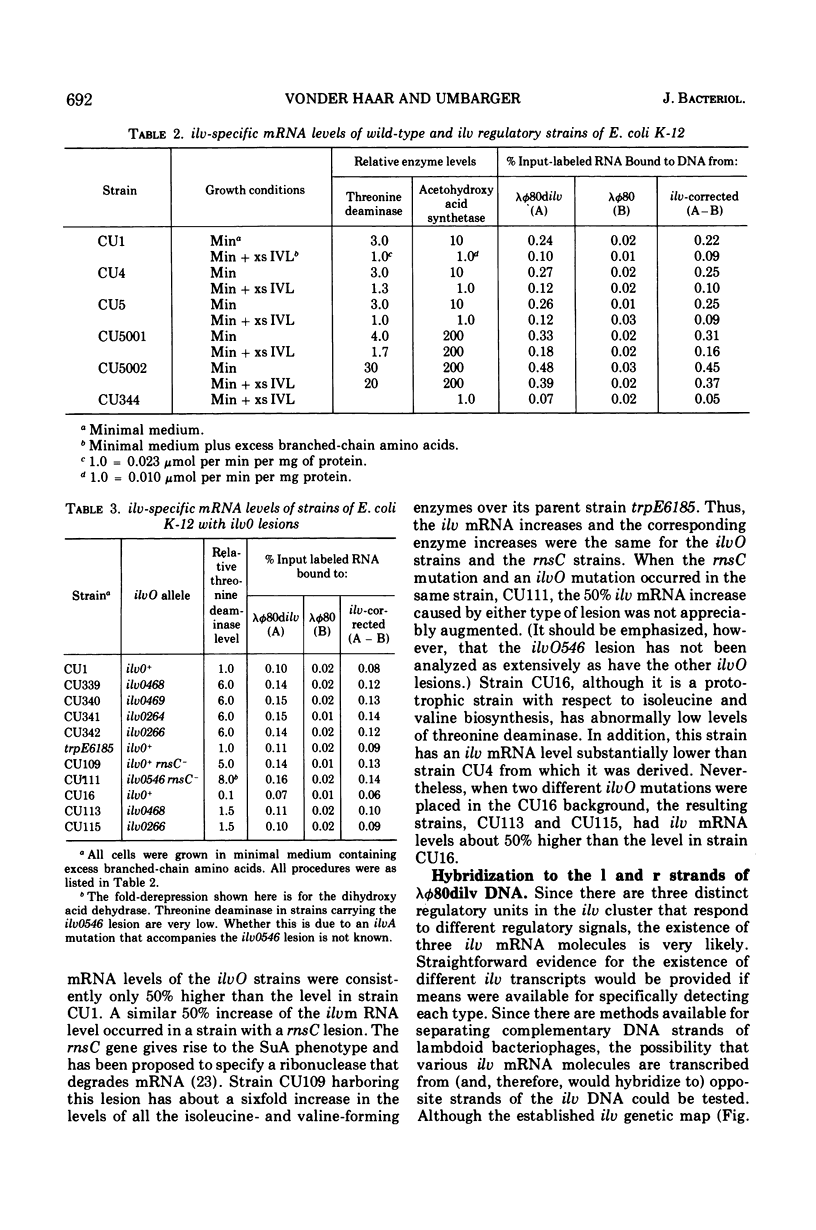
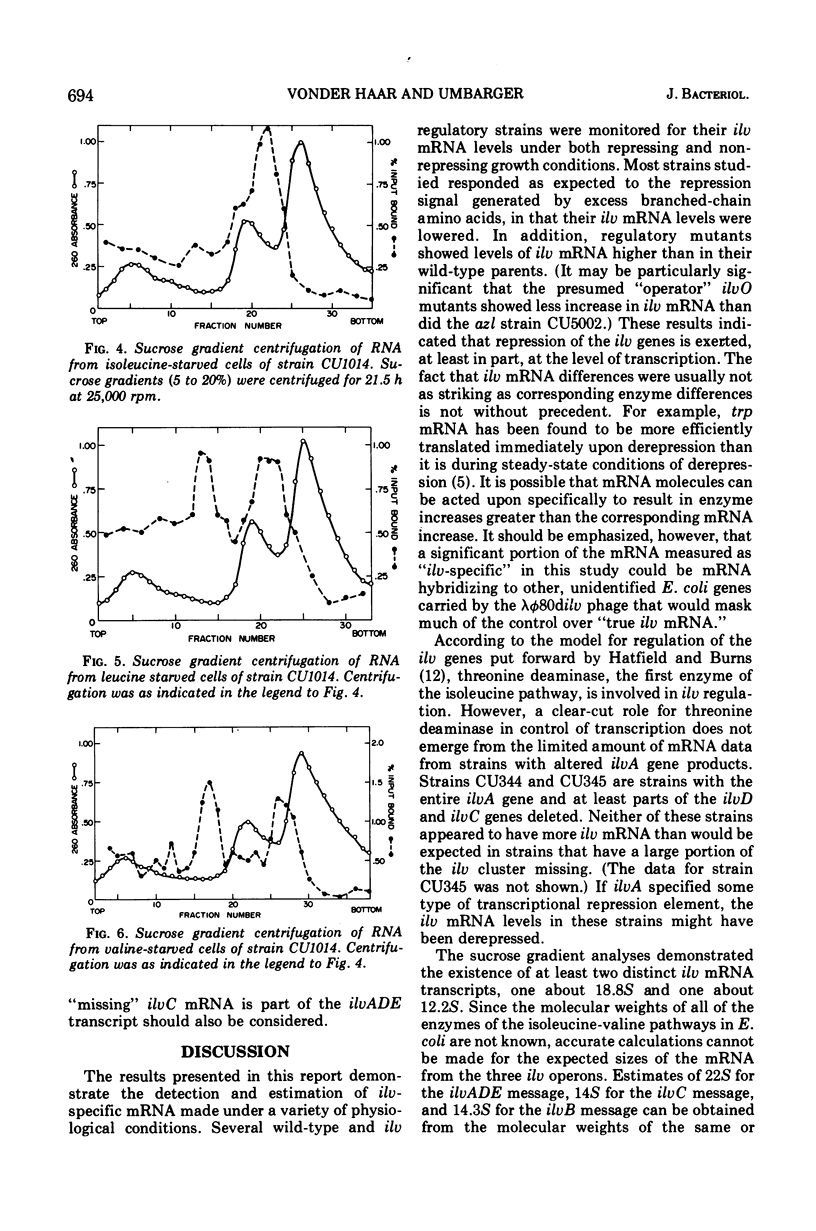
Abstract
Ribonucleic acid-deoxyribonucleic acid (RNA-DNA) hybridization was employed for the determination of messenger RNA transcribed from the ilv gene cluster of Escherichia coli K-12. Strains with derepressed levels of the isoleucine and valine biosynthetic enzymes owing to linked or unlinked genetic lesions were found to exhibit ilv messenger RNA levels from 1.5- to 4-fold higher than did their isogenic parents. When grown under conditions that specifically repressed the synthesis of isoleucine- and valine-forming enzymes, most strains exhibited drastically reduced ilv messenger RNA levels. Hybridization performed with the separated strands of ilv DNA showed that all the ilv genes are transcribed from the same strand, the “l strand” of λφ80CI857St68dilv DNA. Sucrose gradient analyses of RNA extracted from cells starved for isoleucine, valine, or leucine resulted in the detection of at least two distinct types of ilv messenger RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Ratzkin B., Umbarger H. E. The metabolism of valine and isoleucine in Escherichia coli. XVII. The role of induction in the derepression of acetohydroxy acid isomeroreductase. Biochem Biophys Res Commun. 1969 Dec 4;37(6):902–908. doi: 10.1016/0006-291x(69)90216-2. [DOI] [PubMed] [Google Scholar]
- Avitabile A., Carlomagno-Cerillo S., Favvre R., Blasi F. Isolation of transducing bacteriophages for the histidine and isoleucine-valine operons in Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):40–47. doi: 10.1128/jb.112.1.40-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R., Yanofsky C. Transcription initiation frequency and translational yield for the tryptophan operon of Escherichia coli. J Mol Biol. 1972 Aug 14;69(1):89–102. doi: 10.1016/0022-2836(72)90025-3. [DOI] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R. Transposition of the lac region of Escherichia coli. I. Inversion of the lac operon and transduction of lac by phi80. J Mol Biol. 1966 Aug;19(2):254–265. doi: 10.1016/s0022-2836(66)80003-7. [DOI] [PubMed] [Google Scholar]
- Cunin Raymond, Glansdorff Nicolas. Messenger RNA from arginine and phosphoenolpyruvate carboxylase genes in arg R+ and arg R(-) strains of E. coli K-12. FEBS Lett. 1971 Oct 15;18(1):135–137. doi: 10.1016/0014-5793(71)80428-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Echols H. Properties of a mutant blocked in inducibility of messenger RNA for the galactose operon of Escherichia coli. J Mol Biol. 1966 Aug;19(1):38–51. doi: 10.1016/s0022-2836(66)80048-7. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Coleman W. G., Jr, Umbarger H. E. Regulation of isoleucine-valine biosynthesis in an ilvDAC deletion strain of Escherichia coli K-12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1144–1151. doi: 10.1016/0006-291x(74)90816-x. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Mutants of the bacteriophage f2. 8. Control mechanisms for phage-specific syntheses. J Mol Biol. 1966 Aug;19(2):333–348. doi: 10.1016/s0022-2836(66)80008-6. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational repression in the arginine system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1703–1709. doi: 10.1073/pnas.67.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Primakoff P. Relief of polarity in E. coli by "suA". Nature. 1970 Apr 4;226(5240):28–31. doi: 10.1038/226028a0. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., SUGINO Y., NOMURA M. Synthesis and turnover of phage messenger RNA in E. coli infected with bacteriophage T4 in the presence of chloromycetin. J Mol Biol. 1962 Nov;5:527–534. doi: 10.1016/s0022-2836(62)80126-0. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXI. Mutations affecting derepression and valine resistance. J Bacteriol. 1973 Apr;114(1):183–194. doi: 10.1128/jb.114.1.183-194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press R., Glansdorff N., Miner P., De Vries J., Kadner R., Maas W. K. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1971 Apr;68(4):795–798. doi: 10.1073/pnas.68.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
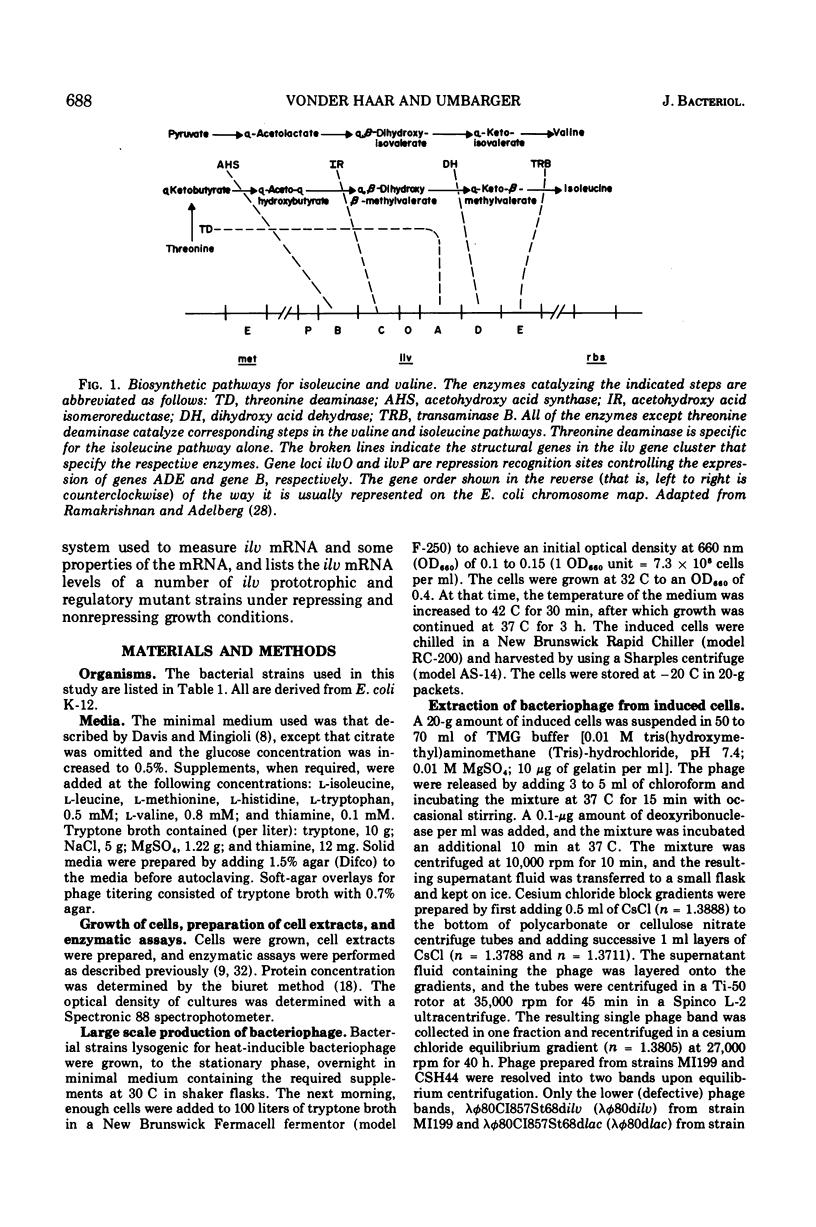
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P., Krzyzek R., Kaden T. M., Arfman E. Effect of arginine and canavanine on arginine messenger RNA synthesis. Biochem Biophys Res Commun. 1971 Sep;44(5):1220–1226. doi: 10.1016/s0006-291x(71)80216-4. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonder Haar R. A., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XIX. Inhibition of isoleucine biosynthesis by glycyl-leucine. J Bacteriol. 1972 Oct;112(1):142–147. doi: 10.1128/jb.112.1.142-147.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Umbarger H. E. Effect of isoleucine, valine, or leucine starvation on the potential for formation of the branched-chain amino acid biosynthetic enzymes. J Bacteriol. 1973 Nov;116(2):548–561. doi: 10.1128/jb.116.2.548-561.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



