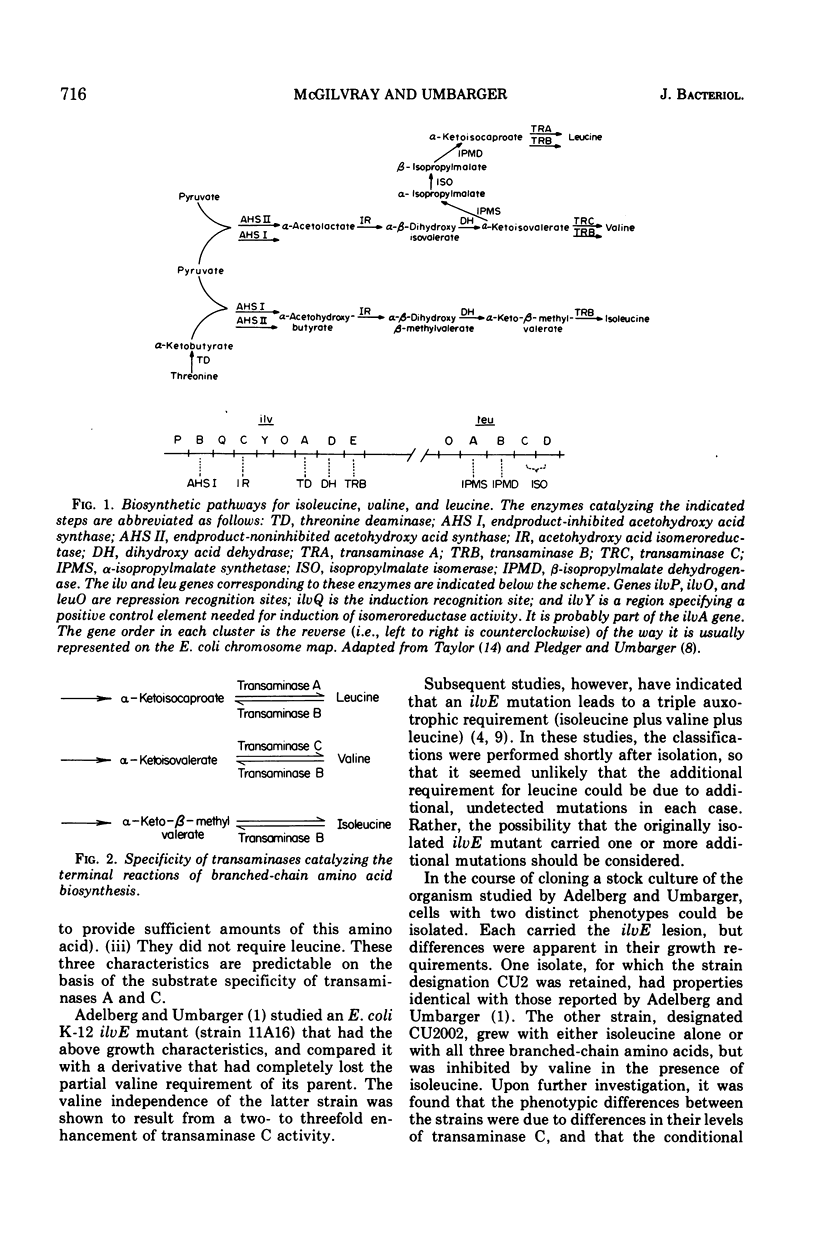
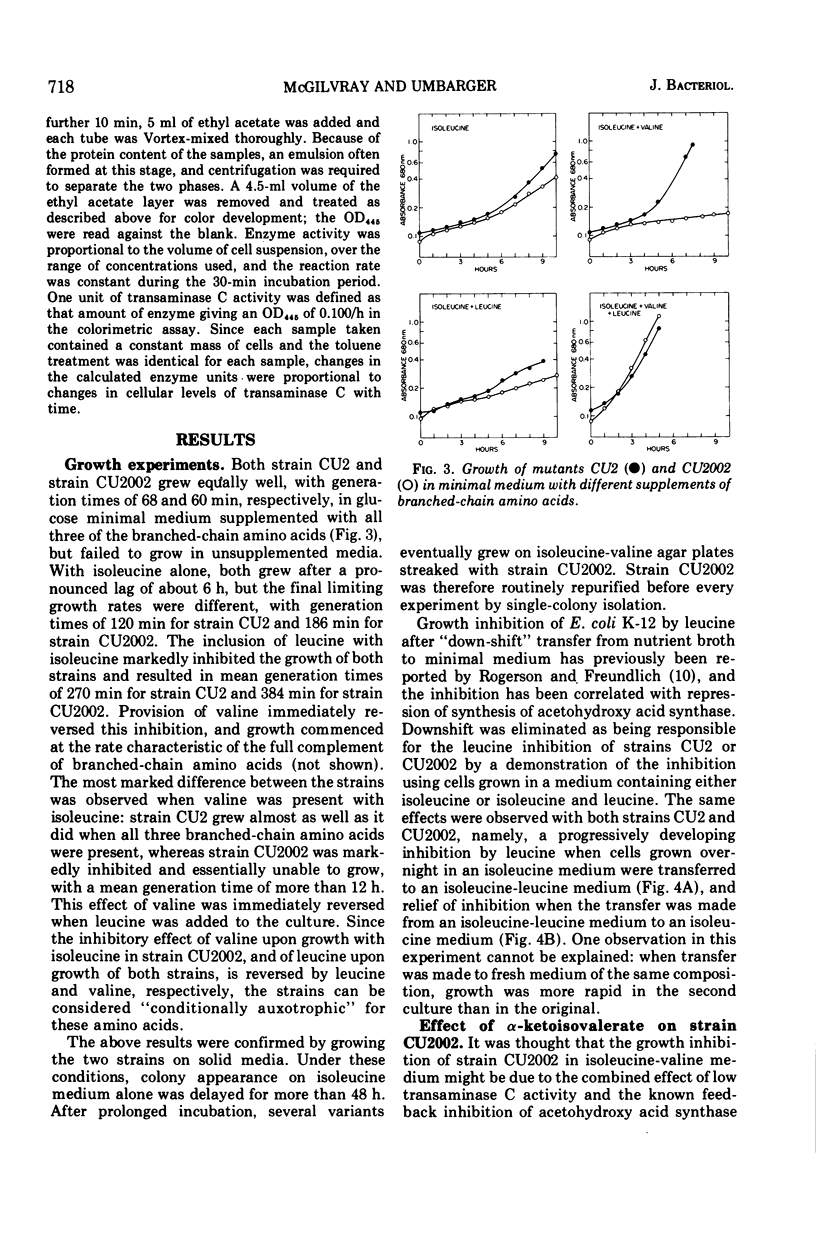
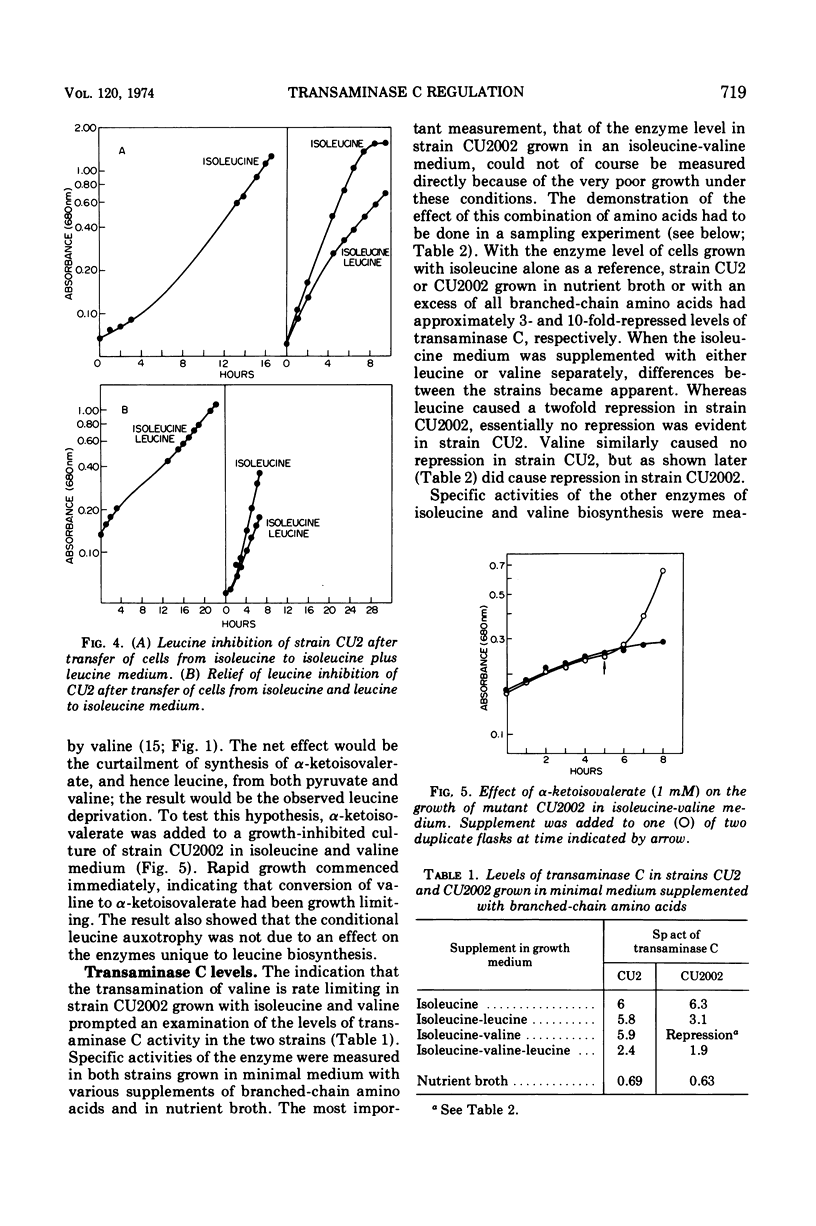
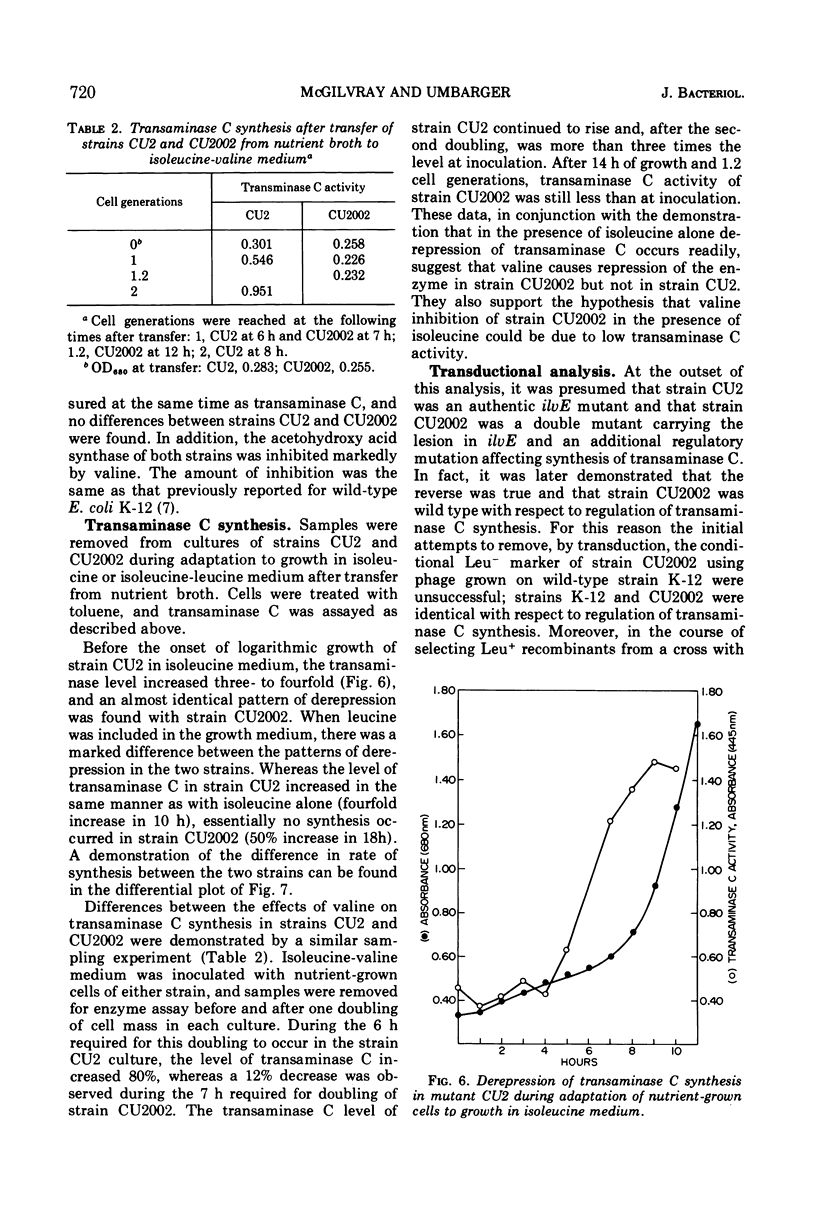
Abstract
The regulation of synthesis of the valine-alanine-α-aminobutyrate transaminase (transaminase C) was studied in Escherichia coli mutants lacking the branched-chain amino acid transaminase (transaminase B). An investigation was made of two strains, CU2 and CU2002, each carrying the same transaminase B lesion but exhibiting different growth responses on a medium supplemented with branched-chain amino acids. Both had the absolute isoleucine requirement characteristic of ilvE auxotrophs, but growth of strain CU2 was stimulated by valine, whereas that of strain CU2002 was markedly inhibited by valine. Strain CU2002 behaved like a conditional leucine auxotroph in that the inhibition by valine was reversed by leucine. Results of enzymatic studies showed that synthesis of transaminase C was repressed by valine in strain CU2002 but not in strain CU2. Inhibition by valine in strain CU2002 appears to be the combined effect of repression on transaminase C synthesis and valine-dependent feedback inhibition of α-acetohydroxy acid synthase activity, causing α-ketoisovalerate (and hence leucine) limitation. The ilvE markers of strains CU2 and CU2002 were each transferred by transduction to a wild-type genetical background. All ilvE recombinants from both crosses resembled strain CU2002 and were inhibited by valine in the presence of isoleucine. Thus, strain CU2 carries an additional lesion that allows it to grow on a medium containing isoleucine plus valine. It is concluded that conditional leucine auxotrophy is characteristic of mutants carrying an ilvE lesion alone.
Full text
PDF

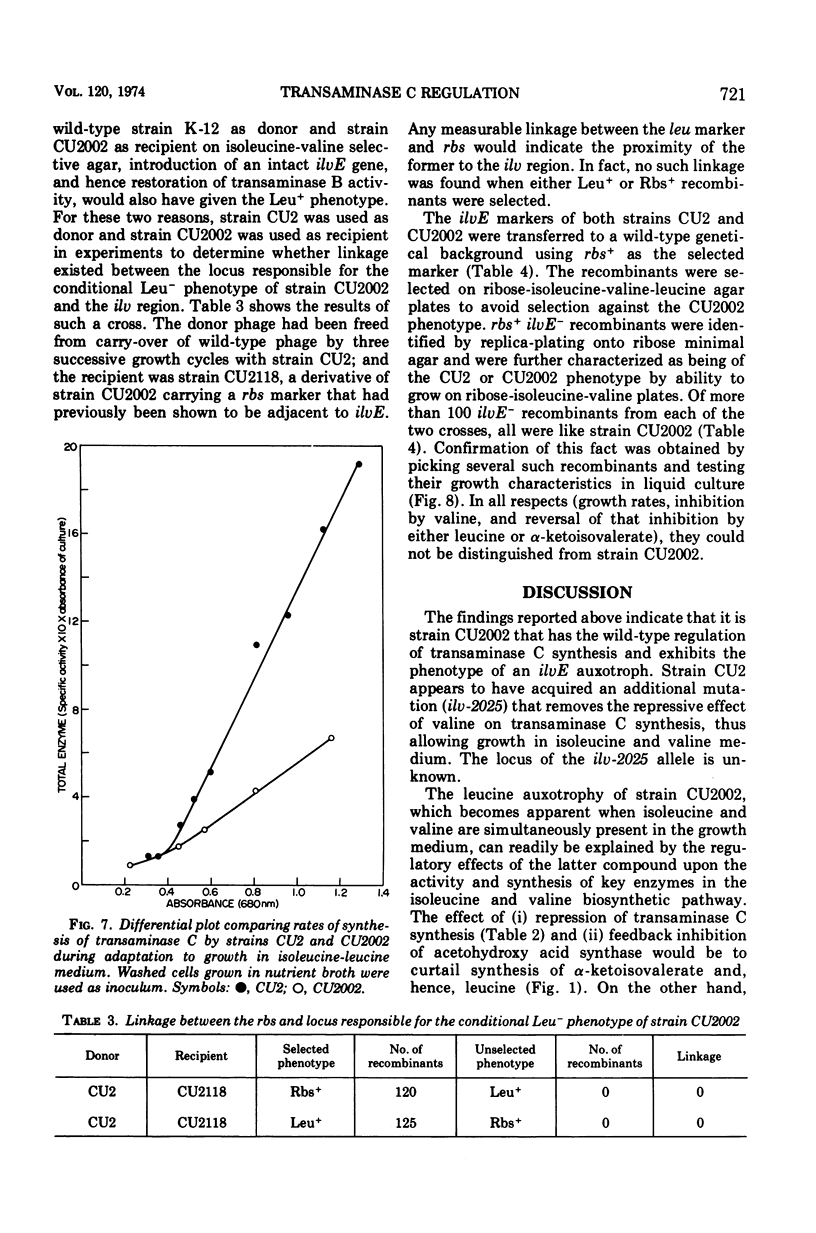
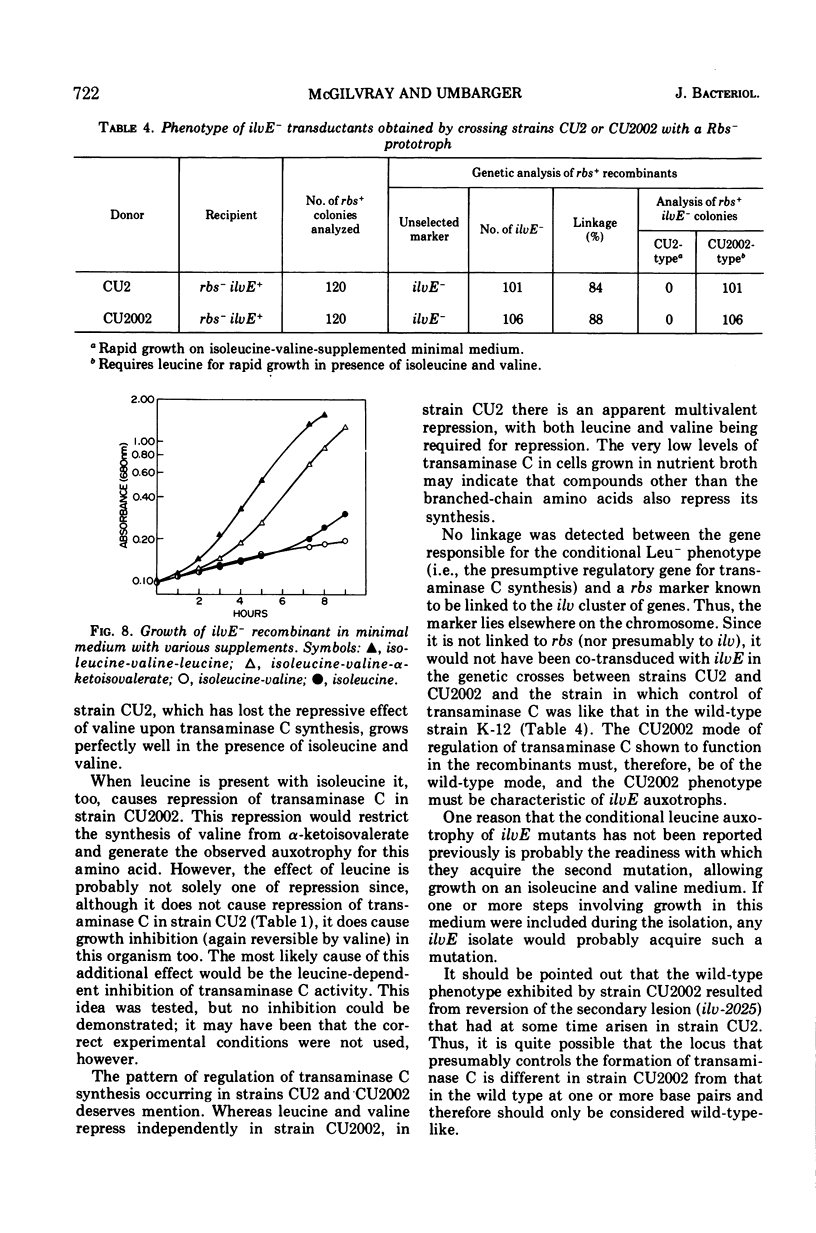

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. V. alpha-Ketoisovaleric acid accumulation. J Biol Chem. 1953 Nov;205(1):475–482. [PubMed] [Google Scholar]
- Collier R. H., Kohlhaw G. Nonidentity of the aspartate and the aromatic aminotransferase components of transaminase A in Escherichia coli. J Bacteriol. 1972 Oct;112(1):365–371. doi: 10.1128/jb.112.1.365-371.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXI. Mutations affecting derepression and valine resistance. J Bacteriol. 1973 Apr;114(1):183–194. doi: 10.1128/jb.114.1.183-194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXII. A pleiotropic mutation affecting induction of isomeroreductase activity. J Bacteriol. 1973 Apr;114(1):195–207. doi: 10.1128/jb.114.1.195-207.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- Rogerson A. C., Freundlich M. Control of isoleucine, valine and leucine biosynthesis. 8. Mechanism of growth inhibition by leucine in relaxed and stringent strains of Escherichia coli K-12. Biochim Biophys Acta. 1970 Apr 14;208(1):87–98. doi: 10.1016/0304-4165(70)90051-6. [DOI] [PubMed] [Google Scholar]
- SILBERT D. F., JORGENSEN S. E., LIN E. C. Repression of transaminase A by tyrosine in Escherichia coli. Biochim Biophys Acta. 1963 Jun 11;73:232–240. doi: 10.1016/0006-3002(63)90307-x. [DOI] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., MUELLER J. H. Isoleucine and valine metabolism of Escherichia coli. I. Growth studies on amino acid-deficient mutants. J Biol Chem. 1951 Mar;189(1):277–285. [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


