Abstract
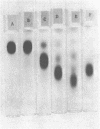
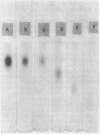
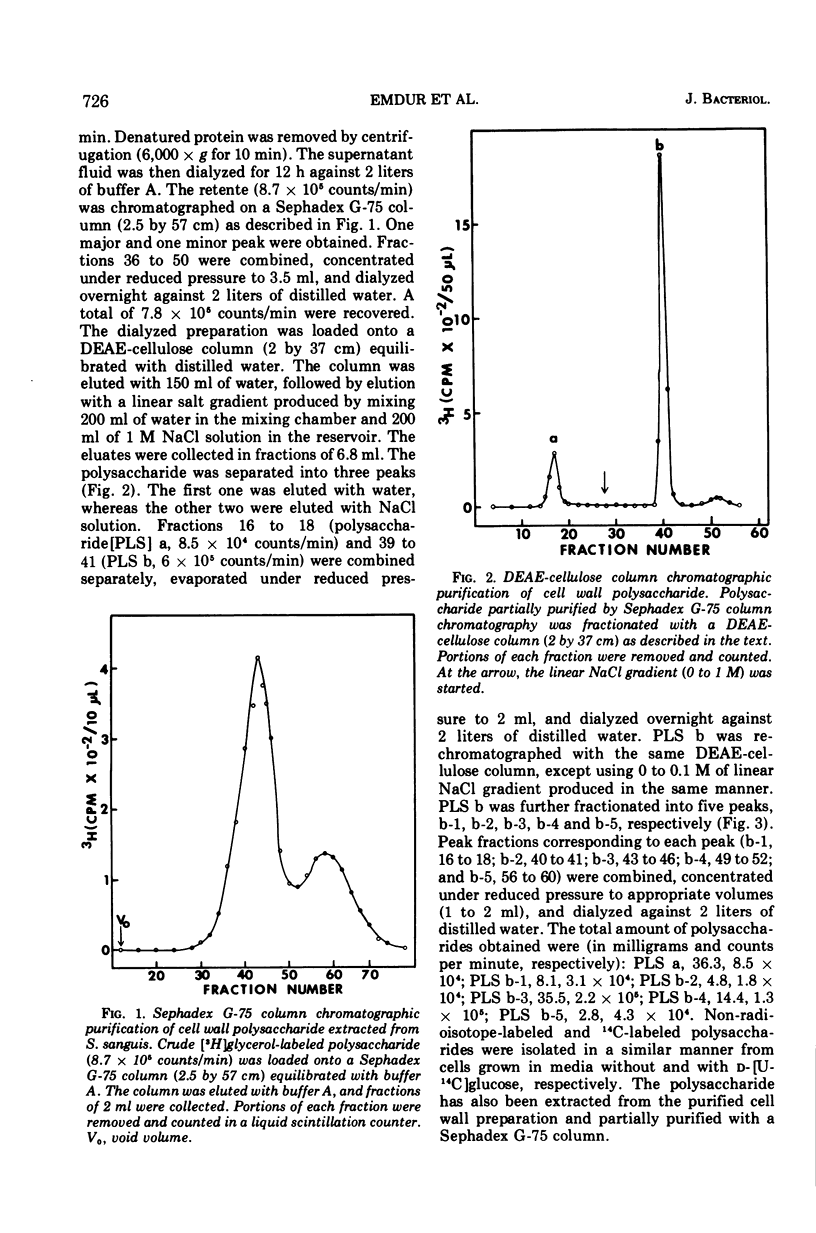
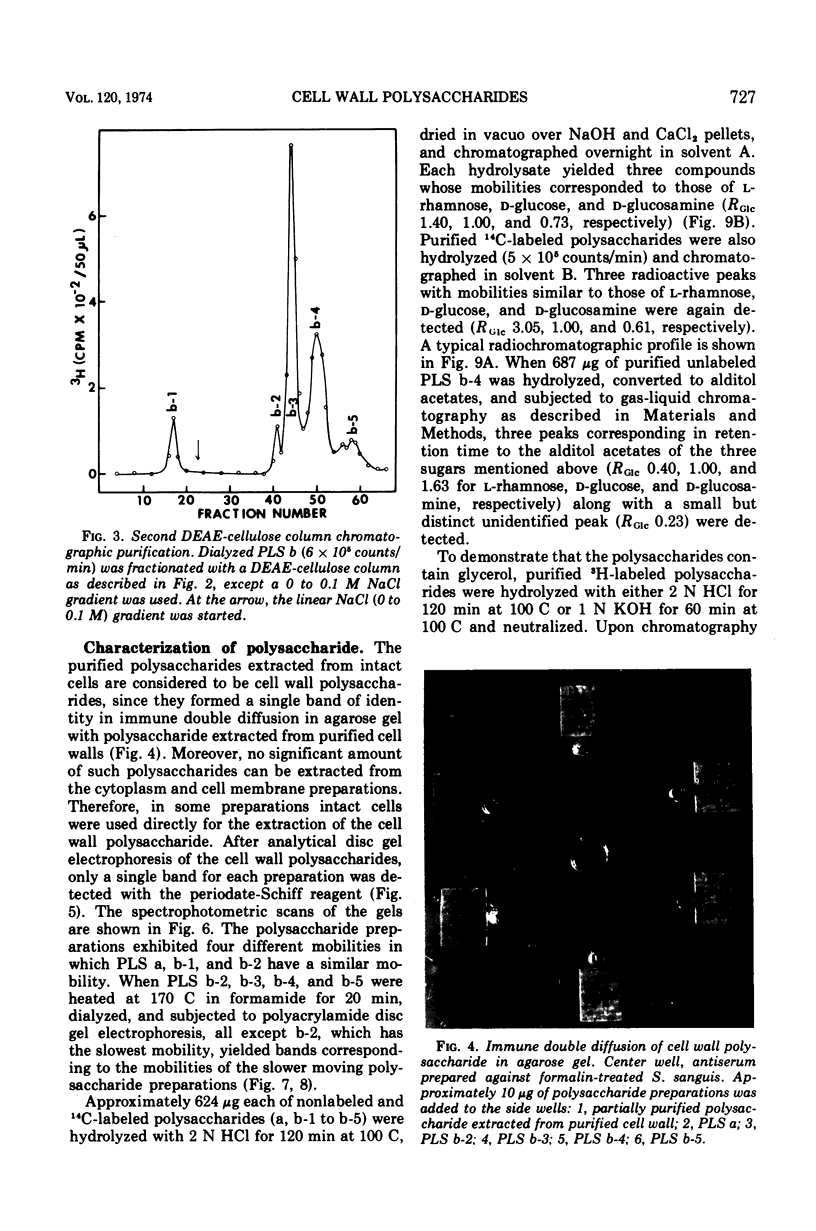
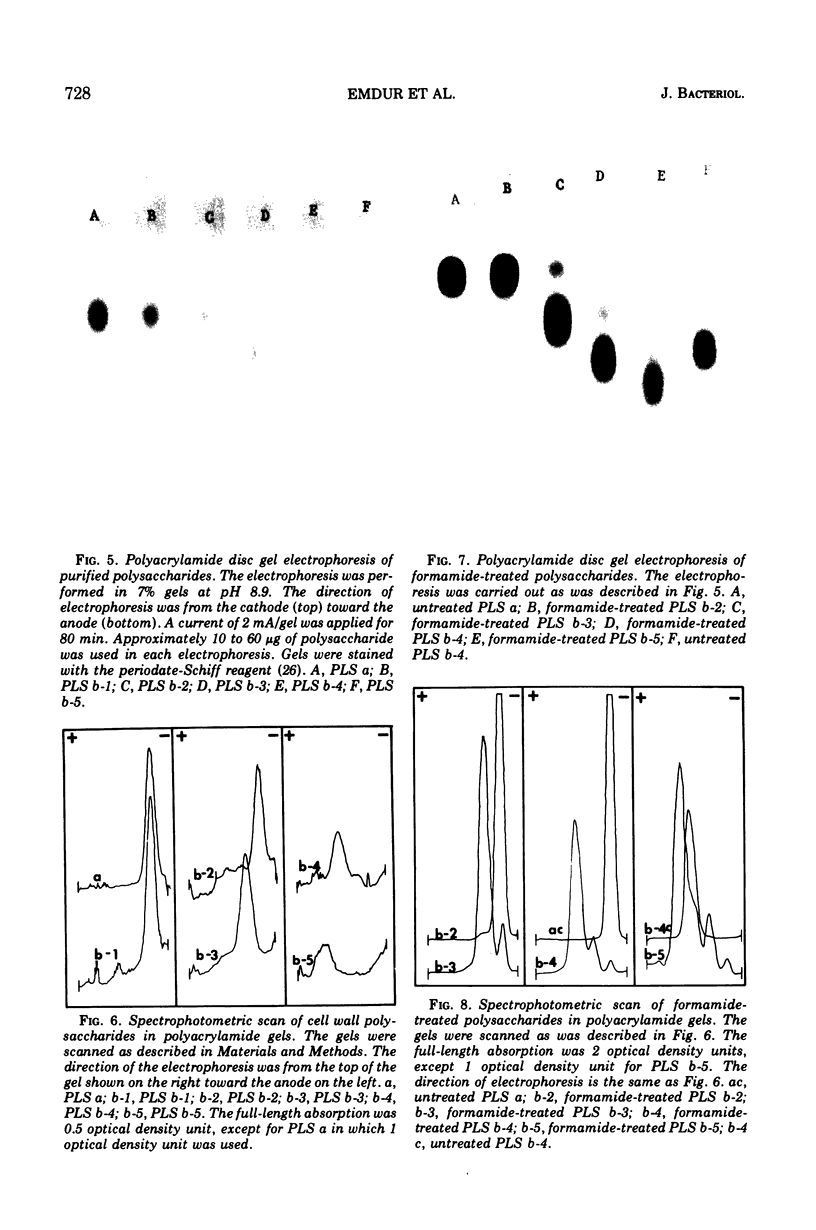
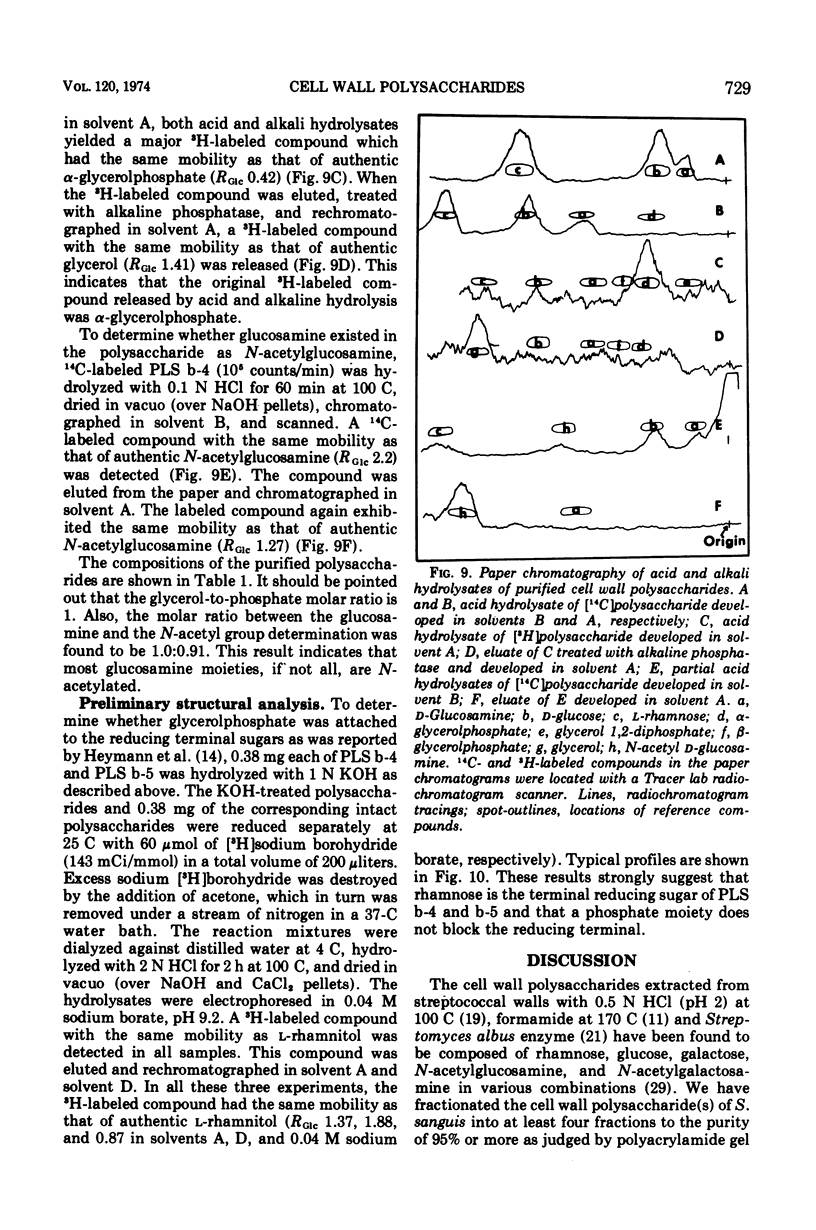
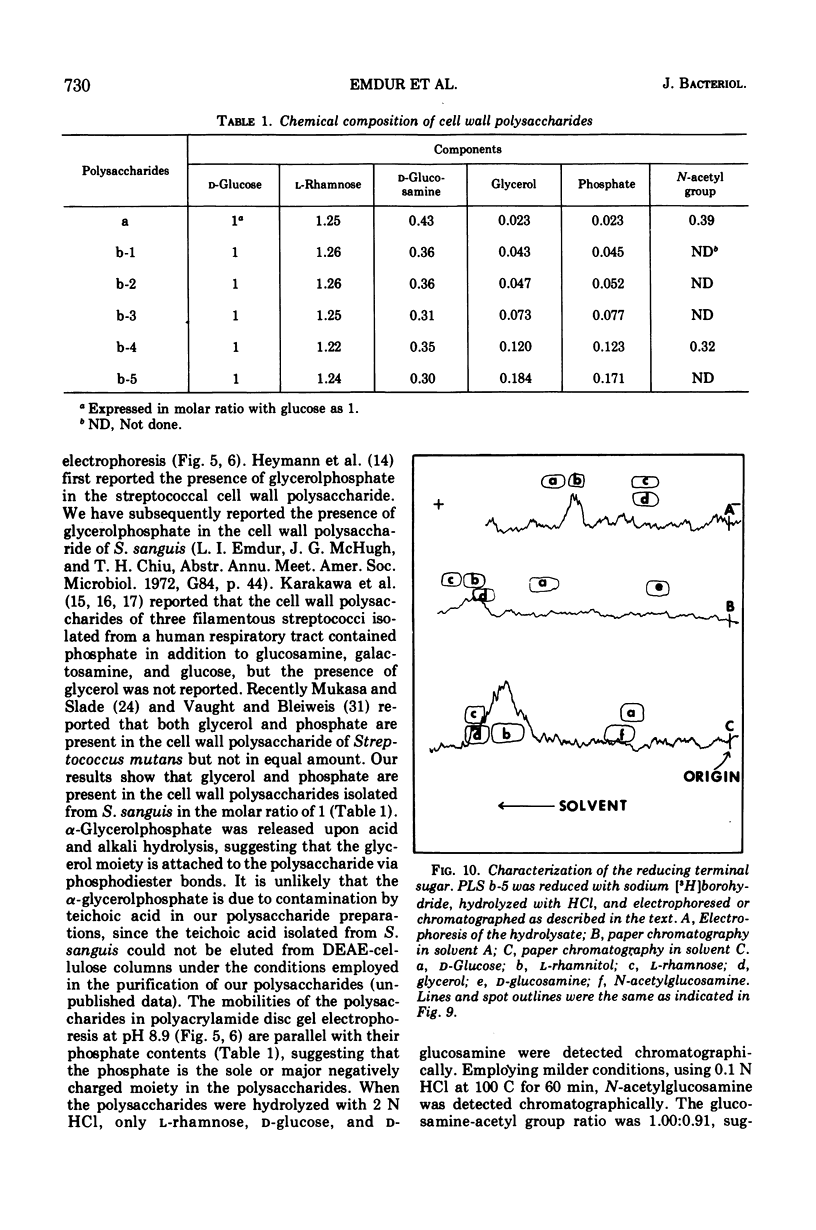
Six glycerolphosphate-containing tetraheteroglycans, a, b-1, b-2, b-3, b-4, and b-5, have been purified from the formamide extracts of Streptococcus sanguis by alcohol and acetone precipitations, Sephadex G-75, and diethylaminoethyl-cellulose column chromatography. The polysaccharides were judged as at least 95% pure by analytical disc gel electrophoresis and immune double diffusion against rabbit antiserum. They were shown to be cell wall polysaccharides, since they formed a single band of identity in immune double diffusion with partially purified polysaccharide extracted from a purified cell wall preparation of S. sanguis. The polysaccharides were composed of l-rhamnose, d-glucose, and N-acetyl d-glucosamine in a similar molar ratio, but varied in their glycerol and phosphate contents. They exhibited four different mobilities in polyacrylamide disc gel electrophoresis at pH 8.9. When they were treated with formamide at 170 C for 20 min, the faster moving polysaccharide(s) yielded polysaccharides with mobilities corresponding to the other slower moving polysaccharides. These results indicate that the polysaccharides originated from the same cell wall polysaccharide and were produced as a result of breakage in the phosphodiester bonds during the formamide extraction procedure. A preliminary structural study shows that the terminal reducing sugar is l-rhamnose and that the glycerol moiety is probably linked to the polysaccharide through a phosphodiester bond.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Burger M. M., Glaser L. The synthesis of teichoic acids. V. Polyglucosylglycerol phosphate and polygalactosylglycerol phosphate. J Biol Chem. 1966 Jan 25;241(2):494–506. [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M. Structure of the walls of Lactobacillus acidophilus strain 63 AM Gasser. Biochemistry. 1970 Jul 21;9(15):2935–2943. doi: 10.1021/bi00817a001. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ELLIOTT S. D. TEICHOIC ACID AND THE GROUP ANTIGEN OF LACTIC STREPTOCOCCI (GROUP N). Nature. 1963 Dec 21;200:1184–1185. doi: 10.1038/2001184a0. [DOI] [PubMed] [Google Scholar]
- Heymann H., Manniello J. M., Barkulis S. S. Structure of streptococcal cell walls. V. Phosphate esters in the walls of group A Streptococcus pyogenes. Biochem Biophys Res Commun. 1967 Feb 21;26(4):486–491. doi: 10.1016/0006-291x(67)90574-8. [DOI] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. 3. Immunochemical studies of the cross-reactivity between the cell wall antigens of a filamentous streptococcus and of pneumococcus. Infect Immun. 1973 Dec;8(6):969–976. doi: 10.1128/iai.8.6.969-976.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. II. Antigenic structure of provisional capsular types 89 and 83-89. Infect Immun. 1973 Dec;8(6):962–968. doi: 10.1128/iai.8.6.962-968.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Buettger C., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. I. Antigenic attributes of provisional capsular type 83 and its relationship to streptococci of so-called group M. Infect Immun. 1973 Dec;8(6):952–961. doi: 10.1128/iai.8.6.952-961.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. A., Buchanan J. G., Baddiley J. The type-specific substance from Pneumococcus type 11A(43). Biochem J. 1969 Oct;115(1):37–45. doi: 10.1042/bj1150037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDOWIEG J., DORFMAN A. A micromethod for the colorimetric determination of N-acetyl groups in acid mucopolysaccharides. Biochim Biophys Acta. 1960 Feb 26;38:212–218. doi: 10.1016/0006-3002(60)91233-6. [DOI] [PubMed] [Google Scholar]
- MCCARTY M. THE ROLE OF D-ALANINE IN THE SEROLOGICAL SPECIFICITY OF GROUP A STREPTOCOCCAL GLYCEROL TEICHOIC ACID. Proc Natl Acad Sci U S A. 1964 Aug;52:259–265. doi: 10.1073/pnas.52.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTY M. The lysis of group A hemolytic streptococci by extracellular enzymes of Streptomyces albus. II. Nature of the cellular substrate attacked by the lytic enzymes. J Exp Med. 1952 Dec;96(6):569–580. doi: 10.1084/jem.96.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCARTY M. The occurrence of polyglycerophosphate as an antigenic component of various gram-positive bacterial species. J Exp Med. 1959 Apr 1;109(4):361–378. doi: 10.1084/jem.109.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Structure and immunological specificity of the Streptococcus mutans group b cell wall antigen. Infect Immun. 1973 Apr;7(4):578–585. doi: 10.1128/iai.7.4.578-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE W. H., FEREBEE S. H., HARRISON H. DISC ELECTROPHORESIS ON POLYACRYLAMIDE GELS OF SERUM MUCOIDS OF INDIVIDUALS WITH SELECTED CHRONIC DISEASES. Ann N Y Acad Sci. 1964 Dec 28;121:460–469. doi: 10.1111/j.1749-6632.1964.tb14217.x. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. J., HORNE R. W. Studies of the bacterial cell wall. II. Methods of preparation and some properties of cell walls. Biochim Biophys Acta. 1951 Jul;7(2):177–197. doi: 10.1016/0006-3002(51)90017-0. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., SLADE H. D. THE CELLULAR LOCATION OF THE STREPTOCOCCAL GROUP D ANTIGEN. J Gen Microbiol. 1964 Dec;37:297–305. doi: 10.1099/00221287-37-3-297. [DOI] [PubMed] [Google Scholar]
- Slade H. D. Extraction of Cell-Wall Polysaccharide Antigen from Streptococci. J Bacteriol. 1965 Sep;90(3):667–672. doi: 10.1128/jb.90.3.667-672.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Kunkel H. G. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J Immunol. 1966 Mar;96(3):464–471. [PubMed] [Google Scholar]
- Vaught R. M., Bleiweis A. S. Antigens of Streptococcus mutans. II. Characterization of an antigen resembling a glycerol teichoic acid in walls of strain BHT. Infect Immun. 1974 Jan;9(1):60–67. doi: 10.1128/iai.9.1.60-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHKO M. E., RICE E. W. Determination of glucose by an improved enzymatic procedure. Clin Chem. 1961 Oct;7:542–545. [PubMed] [Google Scholar]