Abstract
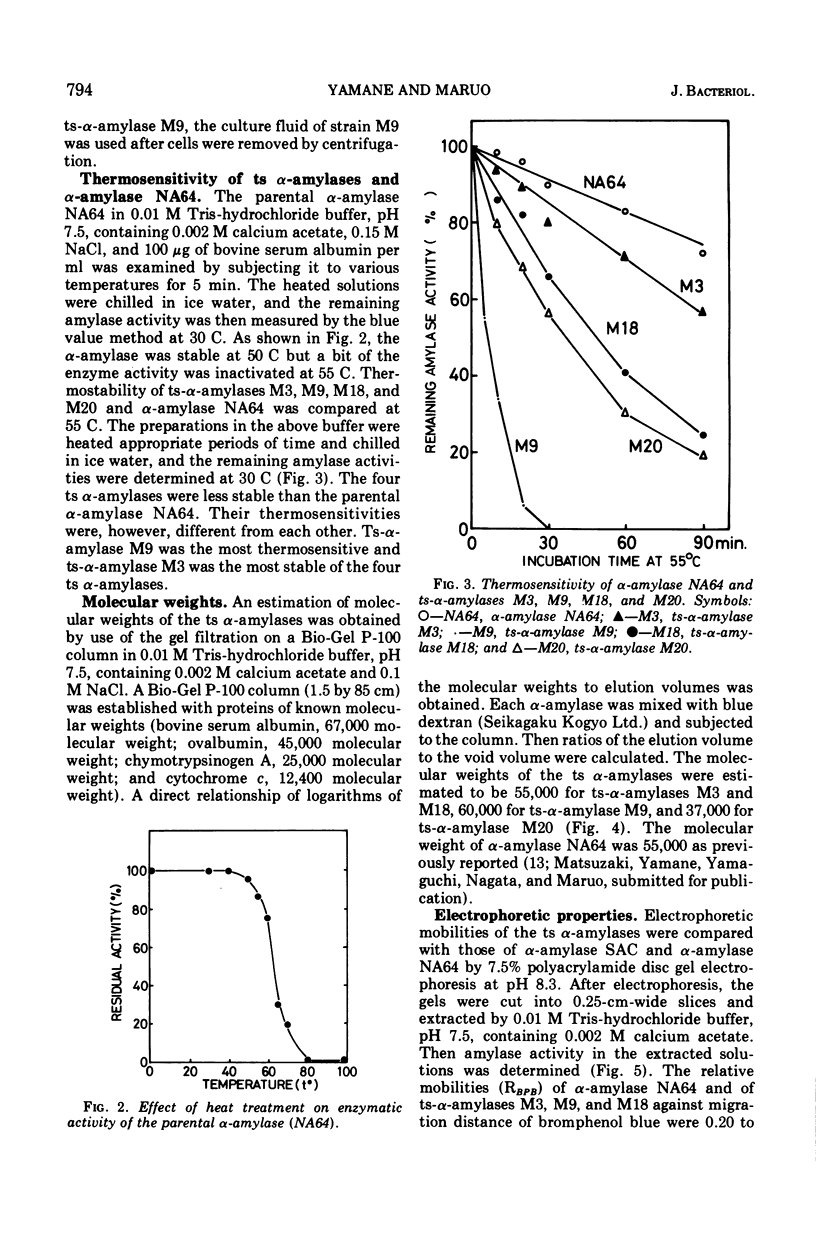
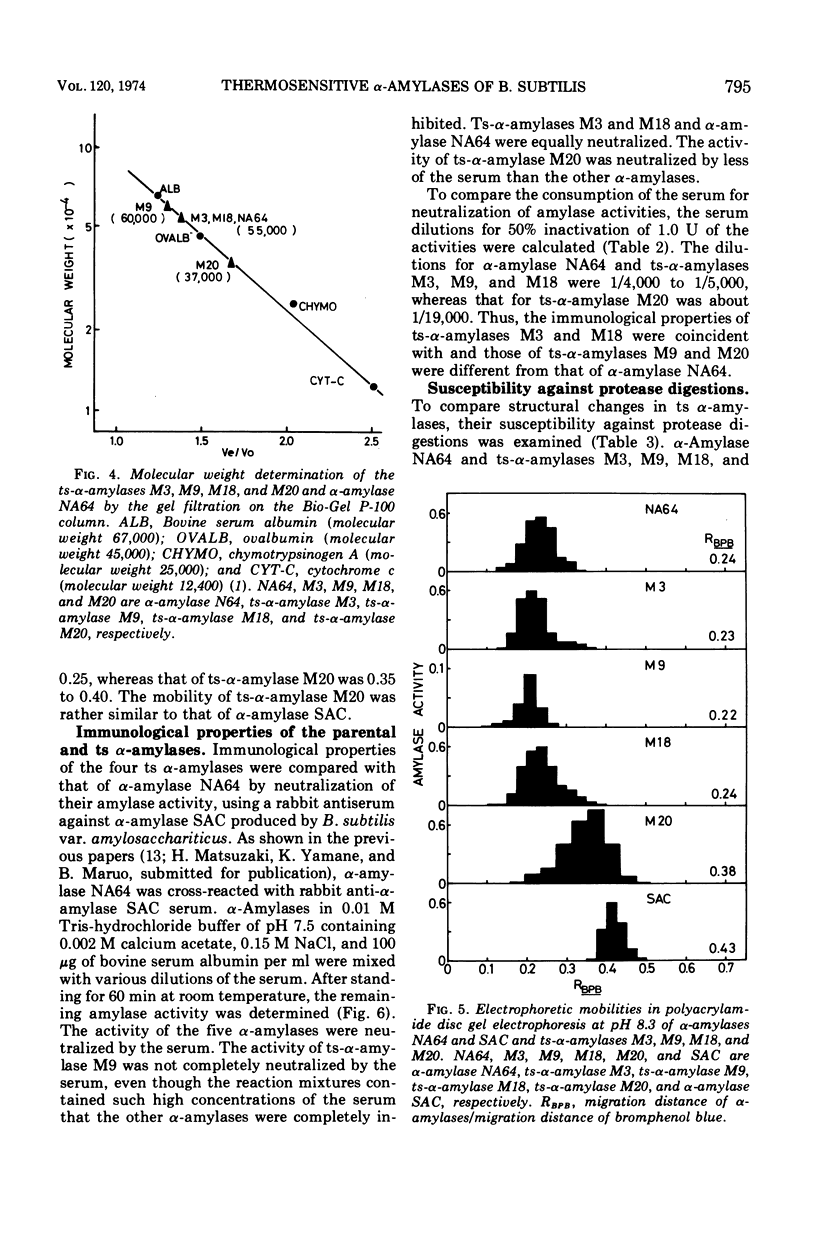
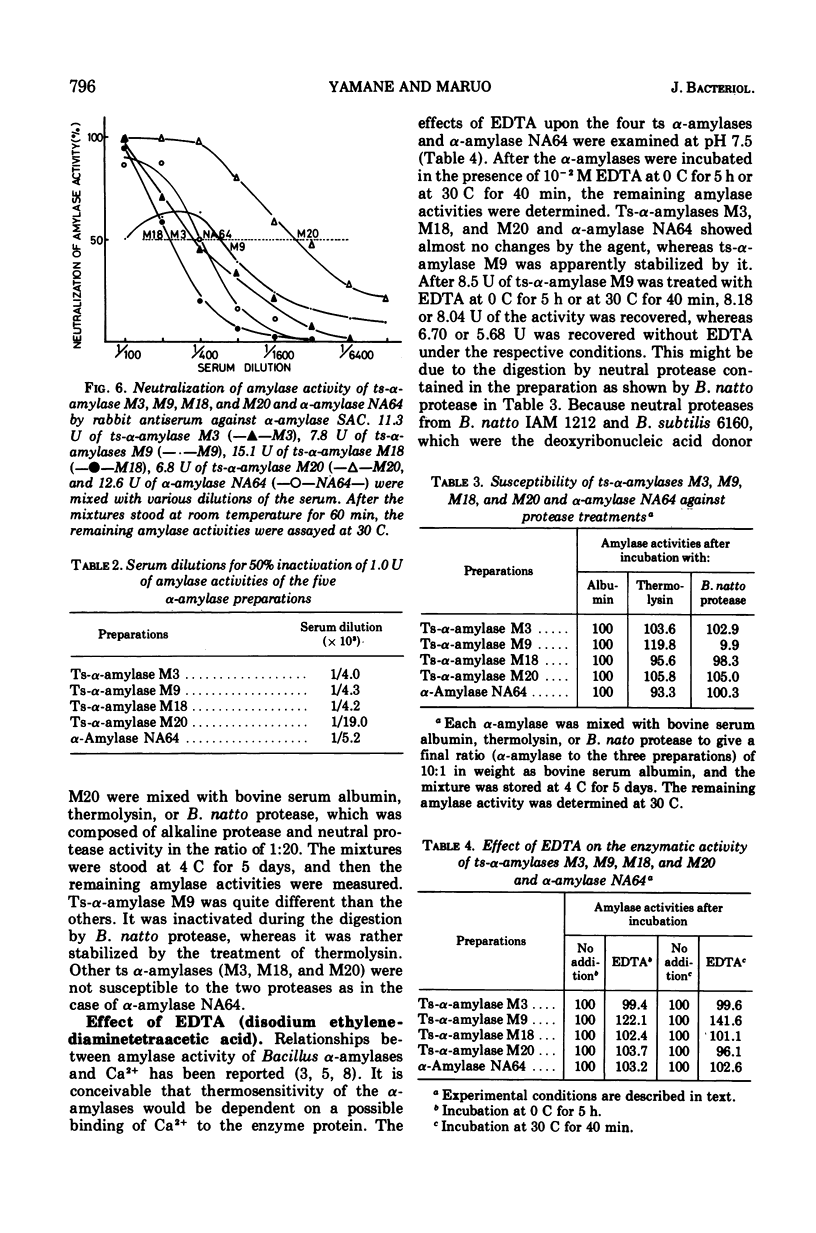
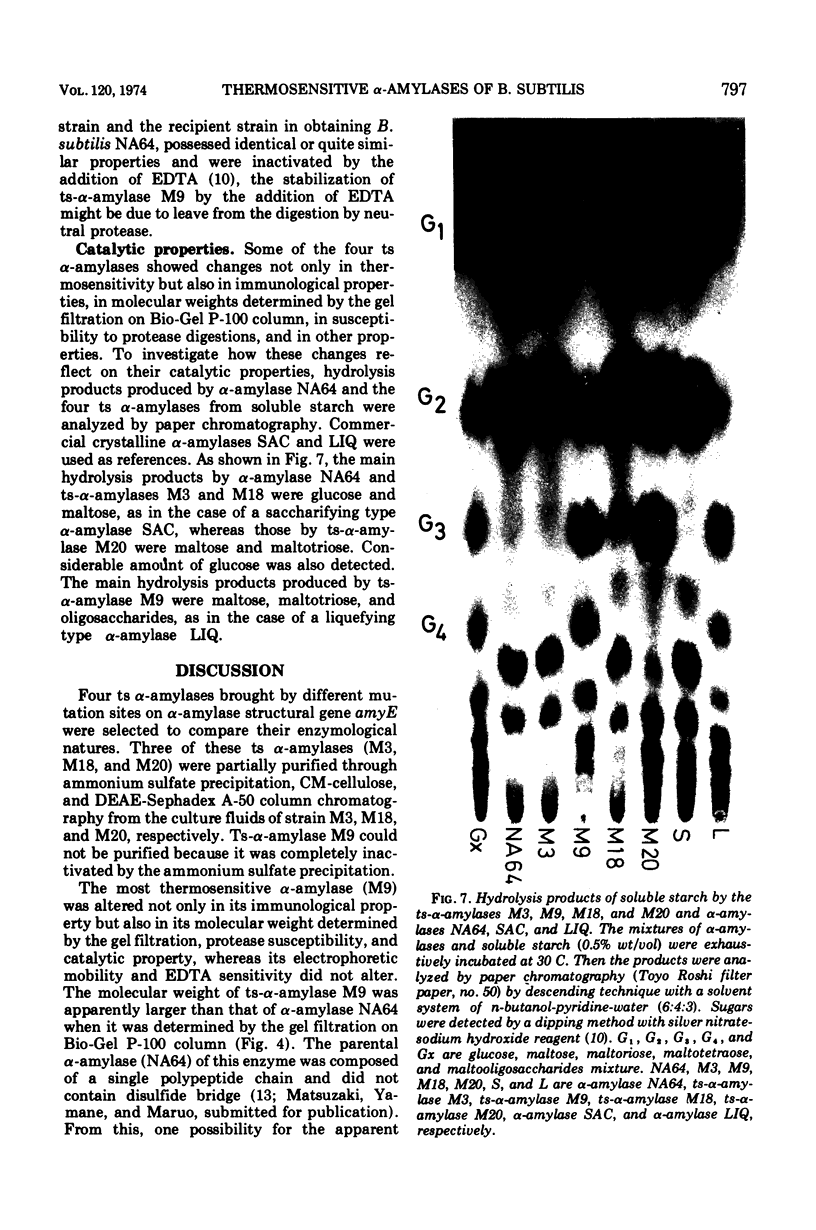
Enzymological properties of four thermosensitive α-amylases (M3, M9, M18, and M20) brought by different mutation sites in α-amylase structural gene of Bacillus subtilis were compared with those of the parental α-amylase NA64. Two thermosensitive α-amylases (M9 and M20) were altered not only in their thermosensitivity but also in their immunological properties, catalytic properties, molecular weights determined by the gel filtration on a Bio-Gel P-100 column, and others. The other two thermosensitive α-amylases (M3 and M18) were altered only in their thermosensitivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kadowaki K., Hosoda J., Maruo B. Effects of actinomycin D and 5-fluorouracil on the formation of enzymes in Bacillus subtilis. Biochim Biophys Acta. 1965 Jun 8;103(2):311–318. doi: 10.1016/0005-2787(65)90170-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitzki A., Reuben J. Abortive complexes of -amylases with lanthanides. Biochemistry. 1973 Jan 2;12(1):41–44. doi: 10.1021/bi00725a007. [DOI] [PubMed] [Google Scholar]
- OISHI M., TAKAHASHI H., MARUO B. Intracellular alpha-amylase in Bacillus subtilis. J Bacteriol. 1963 Jan;85:246–247. doi: 10.1128/jb.85.1.246-247.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Toda H., Narita K. Correlation of the sulfhydryl group with the essential calcium in Bacillus subtilis saccharifying alpha-amylase. J Biochem. 1968 Mar;63(3):302–307. [PubMed] [Google Scholar]
- Uehara H., Yoneda Y., Yamane K., Maruo B. Regulation of neutral protease productivity in Bacillus subtilis: transformation of high protease productivity. J Bacteriol. 1974 Jul;119(1):82–91. doi: 10.1128/jb.119.1.82-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Nagata Y., Maruo B. Isolation of mutants defective in alpha-amylase from Bacillus subtilis: genetic analyses. J Bacteriol. 1974 Aug;119(2):416–424. doi: 10.1128/jb.119.2.416-424.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Yamaguchi K., Maruo B. Purification and properties of a cross-reacting material related to -amylase and biochemical comparison with the parent -amylase. Biochim Biophys Acta. 1973 Jan 25;295(1):323–340. doi: 10.1016/0005-2795(73)90100-1. [DOI] [PubMed] [Google Scholar]