Abstract
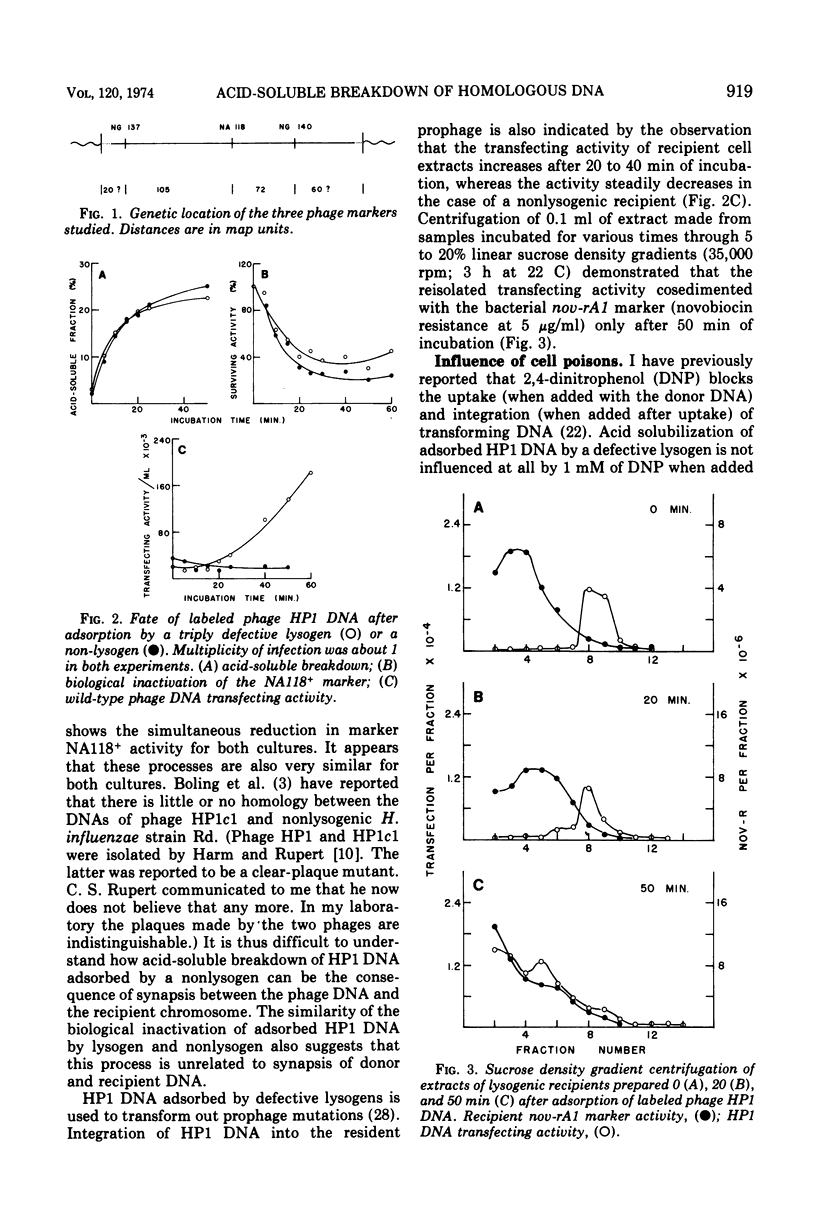
Competent bacteria of Haemophilus influenzae strain Rd were exposed to various kinds of radioactive deoxyribonucleic acid (DNA) for short periods of time and at relatively low temperature. The fate of phage HP1 DNA was studied most extensively. Adsorbed DNA was partially acid solubilized by lysogens and by nonlysogens with very similar kinetics. The biological activity of the DNA decreased extensively in both lysogenic and nonlysogenic recipients. 2,4-Dinitrophenol had no effect on the acid solubilization but largely abolished the biological inactivation. Inactivation kinetics for three different markers and for the triple combination were roughly the same. The presence of 2,4-dinitrophenol in the medium, or the HP1 prophage in the chromosome, did not alter this observation. This suggests that acid solubilization involves the destruction of whole DNA molecules. In view of the absence of DNA homology between phage and host, it is concluded that acid-soluble breakdown of adsorbed transforming DNA is not an integral part of the donor DNA integration process. Behavior of mutant bacteria indicates that neither exonuclease III nor exonuclease V is involved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Determination of inherited traits of H. influenzae by desoxyribonucleic acid fractions isolated from type-specific cells. J Exp Med. 1951 Apr 1;93(4):345–359. doi: 10.1084/jem.93.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Allison D. P., Setlow J. K. Bacteriophage of Haemophilus influenzae. 3. Morphology, DNA homology, and immunity properties of HPlcl, S2, and the defective bacteriophage from strain Rd. J Virol. 1973 Apr;11(4):585–591. doi: 10.1128/jvi.11.4.585-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Conditions affecting the isolation from transformed cells of Bacillus subtilis of high-molecular-weight single-stranded deoxyribonucleic acid of donor origin. J Bacteriol. 1973 Oct;116(1):146–153. doi: 10.1128/jb.116.1.146-153.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Genetic characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Feb;117(2):488–493. doi: 10.1128/jb.117.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S. W., Piekarowicz A. Host specificity of DNA in Haemophilus influenzae: restriction and modification in strain Rd. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1610–1617. doi: 10.1016/0006-291x(72)90793-0. [DOI] [PubMed] [Google Scholar]
- Gunther J. K., Goodgal S. H. An exonuclease specific for double stranded deoxyribonucleic acid. J Biol Chem. 1970 Oct 25;245(20):5341–5349. [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Structure of deoxyribonucleic acid on the cell surface during uptake by pneumococcus. J Bacteriol. 1973 Sep;115(3):1055–1062. doi: 10.1128/jb.115.3.1055-1062.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Transformation and deoxyribonucleic acid size: extent of degradation on entry varies with size of donor. J Bacteriol. 1972 Dec;112(3):1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Allison D. P. Intracellular events during infection by Haemophilus influenzae phage and transfection by its DNA. J Mol Biol. 1973 Apr 25;75(4):581–599. doi: 10.1016/0022-2836(73)90293-3. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUY J. H. Transformability of Haemophilus influenzae. J Gen Microbiol. 1962 Nov;29:537–549. doi: 10.1099/00221287-29-3-537. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Fate of transforming DNA in the Haemophilus influenzae transformation system. J Mol Biol. 1965 Sep;13(2):554–570. doi: 10.1016/s0022-2836(65)80117-6. [DOI] [PubMed] [Google Scholar]
- Stuy J. H., Hoffmann J. F., Duket L. H. Chromosomal recombination in Haemophilus influenzae. Genetics. 1972 Aug;71(4):507–520. doi: 10.1093/genetics/71.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H., Hoffmann J. F. Influence of transformability on the formation of superinfection double lysogens in Haemophilus influenzae. J Virol. 1971 Jan;7(1):127–136. doi: 10.1128/jvi.7.1.127-136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Origin and direction of Haemophilus bacteriophage HP1 DNA replication. J Virol. 1974 Mar;13(3):757–759. doi: 10.1128/jvi.13.3.757-759.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Phage resistance in Haemophilus influenzae. Biochem Biophys Res Commun. 1968 Nov 25;33(4):682–687. doi: 10.1016/0006-291x(68)90350-1. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Prophage mapping by transformation. Virology. 1969 Aug;38(4):567–572. doi: 10.1016/0042-6822(69)90177-9. [DOI] [PubMed] [Google Scholar]
- Stuy J. H., Van der Have B. Degradation of adsorbed transforming DNA by haemophilus influenzae. J Gen Microbiol. 1971 Feb;65(2):147–152. doi: 10.1099/00221287-65-2-147. [DOI] [PubMed] [Google Scholar]
- VOLL M. J., GOODGAL S. H. Recombination during transformation in Hemophilus influenzae. Proc Natl Acad Sci U S A. 1961 Apr 15;47:505–512. doi: 10.1073/pnas.47.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]


