Abstract
Griffonia simplicifolia leaf lectin II (GSII), a plant defense protein against certain insects, consists of an N-acetylglucosamine (GlcNAc)-binding large subunit with a small subunit having sequence homology to class III chitinases. Much of the insecticidal activity of GSII is attributable to the large lectin subunit, because bacterially expressed recombinant large subunit (rGSII) inhibited growth and development of the cowpea bruchid, Callosobruchus maculatus (F). Site-specific mutations were introduced into rGSII to generate proteins with altered GlcNAc binding, and the different rGSII proteins were evaluated for insecticidal activity when added to the diet of the cowpea bruchid. At pH 5.5, close to the physiological pH of the cowpea bruchid midgut lumen, rGSII recombinant proteins were categorized as having high (rGSII, rGSII-Y134F, and rGSII-N196D mutant proteins), low (rGSII-N136D), or no (rGSII-D88N, rGSII-Y134G, rGSII-Y134D, and rGSII-N136Q) GlcNAc-binding activity. Insecticidal activity of the recombinant proteins correlated with their GlcNAc-binding activity. Furthermore, insecticidal activity correlated with the resistance to proteolytic degradation by cowpea bruchid midgut extracts and with GlcNAc-specific binding to the insect digestive tract. Together, these results establish that insecticidal activity of GSII is functionally linked to carbohydrate binding, presumably to the midgut epithelium or the peritrophic matrix, and to biochemical stability of the protein to digestive proteolysis.
Lectins are carbohydrate-binding proteins of nonimmune origin that interact specifically with carbohydrates through direct or water-mediated hydrogen bonding or van der Waals forces. Plant lectins can be grouped into families based on sequence homology and protein topology. One family is composed of proteins that contain one or more 30- to 43-amino acid cysteine-rich chitin-binding domains. Another family, mainly legume lectins, binds carbohydrate substrates by way of interactions involving specific amino acid residues that are located spatially throughout the peptide (1–3). Ca2+ and Mn2+ are required to stabilize the binding site by fixing the position of the amino acid side chains that interact with sugar ligands (4).
Lectins are believed to play a role in the interactions of lectin-producing host plants with other organisms. Rhizobium–legume symbiosis is facilitated evidently by lectins (5, 6). Infection of clover roots by Rhizobium has been linked to the ectopic expression of pea lectin (7) and requires the sugar-binding activity of the protein (8). Evidence is increasing that plant lectins can act also as molecular defenses against insects and other herbivores (9). Larval development of tomato moth Lacanobia oleracea was delayed by expression of snowdrop lectin in potato (10). Similarly, the aphid Acyrthosiphon pisum exhibited high mortality when fed lentil, amaranth, jack bean, or snowdrop lectin (11). The insecticidal activity of plant lectins against many insects, including those in the Coleoptera, Homoptera, and Diptera, has been widely documented in feeding bioassays (11–15).
Little is known about the mode of insecticidal action of plant lectins. In other animal systems, their detrimental effects are attributed mainly to binding of the lectin to the surface of the intestinal epithelial cells. Binding leads to interference with the digestive, protective, or secretory functions of the intestine (16). Bean phytohemagglutinin (PHA) was shown to bind to the brush border cells of the rat small intestine and to disrupt the membrane structure (17). Other deleterious effects of lectin ingestion include inhibition of the brush border enzyme complement in the rat small intestine (18), interference with absorption of various nutrients (19–21), and alteration in the structure and metabolism of epithelial cells. Lectins also induce apoptosis, a programmed sequence of events normally involving the activation of endogenous endonucleases, leading to DNA fragmentation and eventually cell death (22–24). Cell death induced by Con A in murine hepatocytes, on the other hand, is not apoptotic. Rather, it appears to be due to the disruption of the cytoskeleton (25). In all cases however, lectin binding to cell surface receptors is essential for activity.
The antiinsect activity of lectins may be mediated by binding to chitin in the peritrophic matrix or by interacting with glycoproteins on the epithelial cells of the insect midgut. As a result, digestion and assimilation of nutrients presumably is reduced, causing starvation (9, 26, 27). Gatehouse et al. (28) observed strong binding of PHA to midgut epithelium in Callosobruchus maculatus but not in Acanthoscelides obtectus. It was concluded that binding of the lectin to the midgut epithelial cells explained the susceptibility of C. maculatus and the resistance of A. obtectus to PHA. However, Huesing et al. (29) demonstrated later that an α-amylase inhibitor contaminant in the PHA sample rather than PHA itself accounted for the toxicity to C. maculatus. Harper et al. (30), studying European corn borer and Western corn rootworm, tried to establish the relationship between the binding of lectins to insect brush border membranes and their antiinsect activity. However, they found that not all lectins with strong binding activity showed high insecticidal activity. Similarly, not all lectins with negative effects on insects necessarily bound strongly to the insect brush border membrane. Given all of the above, it seems that carbohydrate-binding has not been linked unequivocally to insecticidal activity of lectins.
Several lectins with N-acetylglucosamine (GlcNAc) or Gal/GalNAc specificities have been shown to have insecticidal activity against cowpea bruchid C. maculatus (13). Griffonia simplicifolia lectin II (GSII) is a legume lectin with GlcNAc-binding specificity (31). GSII from seed is a homotetramer and GSII from leaf is a chimerolectin with two different subunits. The large subunit of leaf GSII (rGSII) has the same carbohydrate specificity and antigenicity as the seed GSII (15), whereas the leaf small subunit (sGSII) is a class III chitinase-like protein (32). Both leaf and seed GSII and bacterially expressed rGSII delayed significantly the development of C. maculatus (15). Site-directed mutagenesis was used to identify residues in rGSII essential for GlcNAc-binding activity (33). In the present study, we establish, through bioassays comparing rGSII and variant forms with neutral or GlcNAc-binding null mutations, that GlcNAc binding is required for insecticidal activity of rGSII against the cowpea bruchid. None of the nonbinding mutant proteins had insecticidal activity, whereas all the insecticidal recombinant proteins retained high affinity for GlcNAc. All insect-active proteins bound to cowpea bruchid gut walls, both in vitro and in vivo, whereas the nonbinding proteins failed to do so. The GlcNAc-binding proteins were more resistant to the gut proteolytic activity than were the nonbinding proteins. The biochemical stability of lectins, as well as their ability to bind carbohydrate, thus seems to be prerequisite for their defensive function.
MATERIALS AND METHODS
Expression of rGSII and Mutant Forms in pET28 Vector.
rGSII- and various site-specific mutagenized (D88N, Y134G, Y134D, Y134F, N136D, N136Q, and N196D) rGSII-encoding cDNAs in pET9c were obtained as described (33). The inserts were digested with NdeI and BamHI and ligated into pET28 vector. Escherichia coli (DH5α) was then transformed with the ligation products, and plasmids isolated from these DH5α cells were used to transform bacterial strain BL21 (DE3) for recombinant protein production. Bacterial cells were incubated at 37°C to OD600 of 1.0. Recombinant proteins were induced with isopropyl-d-thiogalactoside overnight at 18°C. Cells were collected by centrifugation, resuspended in binding buffer (20 mM Tris/500 mM NaCl/5 mM imidazole, pH 8.0) and disrupted by sonication (three bursts of 2 min each). Cell debris was removed by centrifugation, and the supernatant was loaded onto a Ni2+ chelate affinity column (Novagen). The column was washed with binding buffer followed by equilibration with thrombin digestion buffer (20 mM Tris/150 mM NaCl/2 mM CaCl2, pH 8.0). Thrombin was added to cleave the recombinant proteins, resulting in their elution from the column (the N-terminal hexahistidine tag section remained bound). After elution, proteins were dialyzed against distilled water for 48 h and lyophilized.
GlcNAc-Binding Activity of rGSII Proteins at Various pH Values.
The rGSII proteins were evaluated for carbohydrate binding by GlcNAc affinity chromatography (EY Laboratories) by using one of the following buffers: 100 mM sodium-phosphate buffer, pH 4.2; 100 mM sodium-acetate buffer, pH 5.5; or 100 mM Tris⋅HCl buffer, pH 7.0 or 8.8. Protein-containing fractions were detected at OD280 and were examined on SDS/PAGE gels.
Preparation of Gut Extracts and Gut Walls of Cowpea Bruchid.
Alimentary tracts from third or fourth instar larvae were dissected into precooled dissection buffer consisting of 200 mM sodium-acetate buffer and 2.5 mM DTT, pH 5.5, as described by Kitch and Murdock (34). Only the region of the alimentary tract anterior to the Malpighian tubules was collected. Pooled guts from 30 larvae were homogenized with a Teflon homogenizer in 100 μl buffer in an Eppendorf tube. The homogenate was centrifuged at 12,000 × g for 5 min, and the supernatant was collected and frozen at −80°C. To prepare gut walls, the guts were slit and rinsed thoroughly in 100 mM NaCl to remove the lumen contents. The gut walls were transferred to a precooled Eppendorf tube containing the same solution and were stored at −80°C.
Cowpea Bruchid Gut Protease Digestion of rGSII Variants.
Gut extracts, prepared as described above, were mixed with recombinant proteins and incubated at 37°C. Samples were collected at 0, 2, 6, and 15 h and were separated on SDS/PAGE gel. BSA was used as a control and the experiment was conducted three times.
Gut Wall Binding of Various Recombinant Proteins.
Pooled gut walls were homogenized gently to minimize individual cell disruption and were centrifuged at 5,000 × g for 5 min. The pellet was resuspended in 100 mM NaCl and recentrifuged. The resuspended pellet was then incubated with 5 μg of recombinant protein for 20 min at room temperature with occasional gentle stirring. After centrifugation, the pellet was resuspended in 100 mM NaCl and washed, as above, two more times. Proteins associated with the pellet were eluted with 100 mM GlcNAc or 100 mM glucose, followed by 100 mM GlcNAc dissolved in 100 mM NaCl. Proteins were separated on SDS/PAGE gels. The experiment was conducted three times.
Bioassay of rGSII Variants Against Cowpea Bruchid.
Recombinant proteins extracted from bacteria and purified by Ni2+ chelate affinity chromatography were mixed with cowpea flour at 4% by weight (35). Ten pellets (about 29 mg per pellet) were made for each treatment by using a mechanical pellet press (Parr Instruments, Moline, IL). One cowpea bruchid egg was placed on each pellet 2 days before hatching. The insects were reared in a growth chamber at 25°C and 60% relative humidity. Within-seed developmental time was the indicator of lectin biological activity.
Collection of Hemolymph, Gut Walls, and Excreta of Cowpea Bruchids Fed on rGSII and Its Mutant Forms.
Artificial seeds (35) containing 2% (wt/wt) rGSII, rGSII-Y134D, rGSII-Y134F, or rGSII-N196D were made and infested with cowpea bruchid eggs as described. The Purdue Insect Feeding Monitor (36) was used to determine the larval instars. Fourth instar larvae were removed from the pellet and cleaned thoroughly in 100 mM NaCl to remove any cowpea flour adhering to the surface of the insect body. After having been blotted on absorbent paper, the larva was placed in 20 μl of the above solution, and the cuticle was sliced opened carefully without injuring the gut. Hemolymph from a single insect mixed with 20 μl of buffer was collected. Each gut wall, free of its lumen contents, was obtained and mixed with 20 μl of protein-loading buffer. In some seeds, larvae were allowed to develop to adults and emerge. From these seeds, cowpea bruchid excreta were collected and mixed with 20 μl of protein-loading buffer as well. Five microliter of hemolymph, 2 μl of gut walls, and 2 μl of excreta extracts from each individual insect or pellet were examined for rGSII proteins by using anti-rGSII antibody and immunoblots. There were three to six individual insects or pellets for each treatment. A duplicate gel was used for visualization of total proteins.
RESULTS
Expression, Purification, and GlcNAc-Binding of rGSII Proteins.
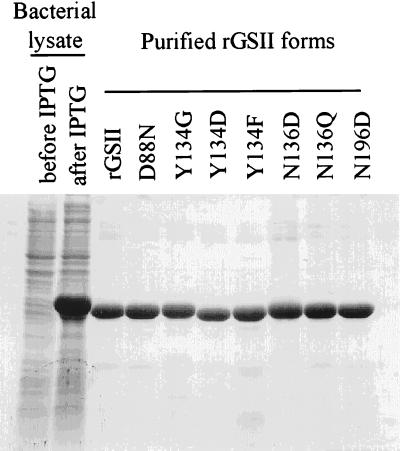
cDNAs encoding rGSII or mutant variants (rGSII-D88N, rGSII-Y134G, rGSII-Y134D, rGSII-Y134F, rGSII-N136D, rGSII-N136Q and rGSII-N196D) were cloned into the pET28 vector and were expressed as fusion proteins with a hexahistidine tag at the N terminus (Table 1). Because mutations D88N, Y134G, Y134D, N136D, and N136Q eliminated GlcNAc-binding capacity of rGSII (33), all proteins were purified by Ni2+ chelate chromatography and were subjected to thrombin digestion to remove the hexahistidine tag (Fig. 1).
Table 1.
GlcNac-binding affinity of rGSII variants at different pH
| Protein | pH 4.2 | pH 5.5 | pH 7.0 | pH 8.8 |
|---|---|---|---|---|
| rGSII | ++ | ++ | ++ | ++ |
| rGSII-D88N | − | − | − | − |
| rGSII-Y134G | − | − | − | − |
| rGSII-Y134D | − | − | − | − |
| rGSII-Y134F | ++ | ++ | ++ | ++ |
| rGSII-N136D | + | + | − | − |
| rGSII-N136Q | − | − | − | − |
| rGSII-N196D | ++ | ++ | ++ | ++ |
++, strong affinity for GlcNAc; +, some affinity for GlcNAc; −, no affinity for GlcNAc.
Figure 1.
Purification of rGSII and its mutant forms by Ni2+ chelate affinity chromatography. Transformed host E. coli strain BL21 (DE3) cells were used for recombinant protein production, followed by protein purification with a Ni2+ chelate affinity column (Novagen).
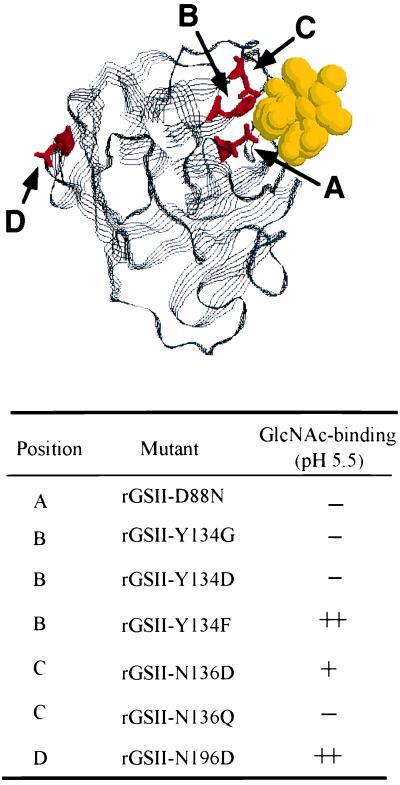
The physiological pH of C. maculatus larval midguts has been determined to be about 6.1 by using a pH microelectrode (34). However, the midgut contains a thiol-dependent protease with pH optimum at 5.0 (34). Accordingly, GlcNAc binding of the recombinant proteins was assessed at pH 4.2, 5.5, 7.0, and 8.8 (Table 1). Results confirmed that residues Asp88, Tyr134, and Asn136 are essential for carbohydrate binding of rGSII (33). Interestingly, the rGSII-N136D exhibited some GlcNAc-binding affinity at pH 4.2 and pH 5.5, but had no affinity for the carbohydrate at neutral or basic pH (Table 1).
The conserved tertiary structure of legume lectins is composed of two antiparallel pleated sheets connected by loops and β-turns, with metal- and carbohydrate-binding sites that occupy similar positions (1). Furthermore, the crystallographic structure of a homologous G. simplicifolia lectin, GSIV, interacting with its carbohydrate ligand has been determined (37). Based on this model, we simulated a mechanism by which rGSII interacts with GlcNAc (Fig. 2). This model predicts that Asp88, Tyr134, and Asn136 are in close contact with the carbohydrate substrate, so mutations to these residues should substantially affect carbohydrate binding. Asn196 is well outside the binding region, so it is not surprising that mutation at this position did not alter carbohydrate binding.
Figure 2.
Topological orientation of rGSII residues in the interaction with GlcNAc. This simulation is modeled from the tertiary structure of GSIV, a closely related legume lectin, complexed with the carbohydrate. The letters illustrate mutated residues in rGSII.
Insecticidal Activity and Biochemical Stability of rGSII Proteins.
Within-seed development time of cowpea bruchid was prolonged significantly when rGSII and variants (rGSII-Y134F and rGSII-N196D) that retained GlcNAc-binding activity were incorporated into artificial seeds (Table 2). Proteins containing mutations that eliminated carbohydrate binding (D88N, Y134G, Y134D, and N136Q) were no longer insecticidal. The mutant protein with intermediate GlcNAc-binding activity (rGSII-N136D) exhibited intermediate insecticidal activity.
Table 2.
Insecticidal activity of rGSII variants is correlated with their carbohydrate-binding activity
| Treatment | Insecticidal activity WSDT, days* | GlcNAc-binding, pH 5.5 |
|---|---|---|
| thrombin | 30.8 a | |
| control | 31.1 a | |
| BSA | 31.5 a | |
| rGSII-Y134D | 34.3 a b | − |
| rGSII-N136Q | 34.7 a b | − |
| rGSII-D88N | 36.3 b | − |
| rGSII-Y134G | 37.4 b | − |
| rGSII-N136D | 38.3 b c | + |
| rGSII-N196D | 44.5 c d | ++ |
| rGSII-Y134F | 45.9 c d | ++ |
| rGSII | 53.0 d | ++ |
Within-seed developmental time (WSDT) means followed by the same letter are not significantly different (P = 0.05).
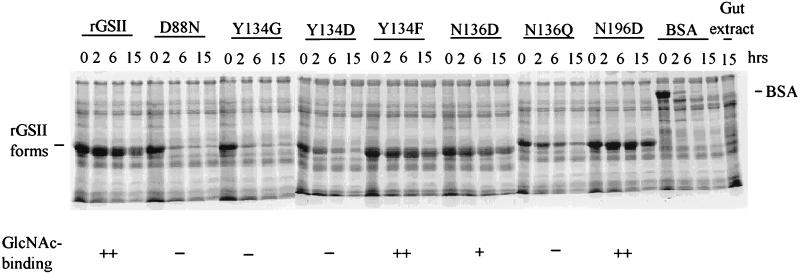
Insecticidal and GlcNAc-binding activities were correlated with biochemical stability when the proteins were incubated with gut extracts of third or fourth instar larvae (Fig. 3). Proteolysis of the rGSII-Y134D or BSA was reduced substantially by trans-epoxysuccinyl-l-leucylamido (4-guanidino)-butane (E-64), an irreversible inhibitor of cysteine proteases (data not shown). This result is in accordance with earlier evidence indicating that cysteine proteases are the major protein digestive enzymes used by cowpea bruchid (34, 38). The rGSII protein did not prevent proteolysis of BSA by C. maculatus gut extract (data not shown), indicating that biochemical stability and insecticidal activity of rGSII is not due to inherent protease inhibitor activity.
Figure 3.
Insecticidal and GlcNAc-binding rGSII proteins are resistant to hydrolysis by insect midgut proteases. Gut extract supernatant, obtained as described in Materials and Methods, was mixed with each recombinant protein, incubated at 37°C for 0, 2, 6, and 15 h and examined by SDS/PAGE. Degradation of BSA was used as a positive control for protease activity of the extract.
Insecticidal rGSII Proteins Associate with GlcNAc Targets in the Digestive Tract of Cowpea Bruchid.
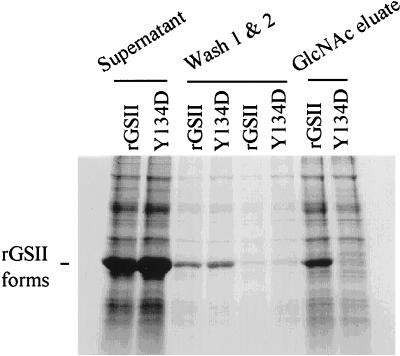
The GlcNAc-binding and insecticidal rGSII bound to a cowpea bruchid gut wall preparation in vitro (Fig. 4). The same results were obtained with rGSII-Y134F and rGSII-N196D (data not shown). A small proportion of the bound rGSII variants could be eluted by glucose (data not shown), presumably reflecting nonspecific binding. However, most of the bound protein could be eluted only with GlcNAc, indicating the presence of GlcNAc-containing ligand(s) for lectin binding. The mutant form that could no longer bind GlcNAc (rGSII-Y134D) did not associate with the gut wall.
Figure 4.
rGSII associates specifically with a GlcNAc substrate in cowpea bruchid gut wall extract in vitro. Isolated alimentary tracts from third or fourth instar larvae were washed free of gut contents. The gut walls were homogenized gently and centrifuged. The gut wall pellet was incubated with rGSII or rGSII-Y134D and was centrifuged again to remove excess or unbound proteins. Proteins associated with the gut walls were eluted with GlcNAc. The above fractions were examined on SDS/PAGE.
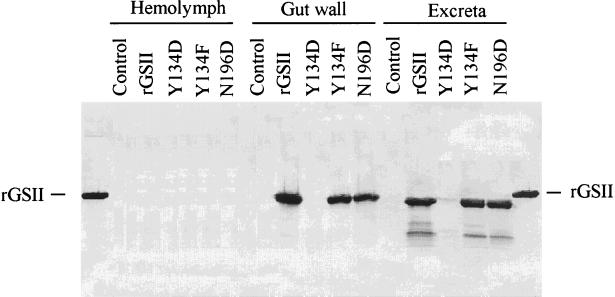
Hemolymph, gut walls, and excreta were collected from cowpea bruchid larvae reared on bioassay diet containing rGSII or its variant proteins. GlcNAc-binding proteins (rGSII, rGSII-Y134F, and rGSII-N196D) associated specifically with the gut walls and excreta but not with the hemolymph, whereas the GlcNAc-binding null mutant protein (rGSII-Y134D) could not be detected in any fraction (Fig. 5). The rGSII proteins were not detected in the hemolymph, so their effect on C. maculatus appears to involve action in or on the midgut. The rGSII proteins that were detected in gut walls and in excreta migrated further down the SDS gel than did native rGSII, indicating proteolytic cleavage by the gut proteases. However, these processed proteins retained the capacity to bind GlcNAc (data not shown). Thus, the tertiary structure of these proteins is not affected adversely by protease digestion, or the small fragment deleted is not necessary for binding activity. Failure to detect rGSII-Y134D in any fraction reflects its rapid rate of proteolysis in the insect gut.
Figure 5.
rGSII proteins interact specifically with cowpea bruchid gut walls in vivo and pass through the gut with minimal hydrolysis. Artificial seeds containing rGSII, rGSII-Y134D, rGSII-Y134F, or rGSII-N196D were infested with cowpea bruchid eggs. Insects feeding on cowpea flour only were used as negative controls for reaction with anti-rGSII antibody. Fourth instar larvae were removed from the pellets. Hemolymph, gut walls, and excreta of cowpea bruchid were collected as described in Materials and Methods. rGSII and its mutant forms present in hemolymph, gut walls, and excreta were detected by anti-rGSII antibody by using immunoblots.
DISCUSSION
Specific carbohydrate-binding activity distinguishes lectins from other plant proteins. Such binding enables them to recognize and attach to glycoconjugates present in other organisms, such as bacteria, fungi, insects, and mammals. Because glycoproteins are major constituents of insect digestive tract membranes, it is plausible that the insect gut contains specific ligand-binding molecules that are targets for plant defensive lectins. Our data indicate that GlcNAc binding is essential for the insecticidal activity of GSII. In vitro as well as in vivo experiments provided evidence that recombinant proteins with affinity for GlcNAc bound to the gut walls, whereas rGSII-Y134D, which had lost this affinity, did not. The binding sites were largely GlcNAc specific, although a small proportion of nonspecific low-affinity binding was also detected. Eisemann et al. (27) showed that wheat germ agglutinin binds to both peritrophic matrix and epithelial cells of the blow fly Lucilia cuprina. They suggested that this binding decreased the absorption of nutrients, which accounted for the poor growth and development of L. cuprina larvae-fed wheat germ agglutinin. Our experiments support this hypothesis, i.e., the delay in development caused by GSII involves binding of the lectin to the midgut epithelial cells and disruption of cell function, with reduced rates of digestion and absorption. In rats, numerous studies have investigated how lectins affect cellular morphology and metabolism of the small intestine. Lectins bind apparently to cells lining the intestine and are then endocytosed. Binding and endocytosis induce enhanced metabolic activity of the epithelial cells and leads to hyperplasia and hypertrophy of the small intestine, with substantially increased DNA, RNA, and protein syntheses (16). In insects, GSII bound to the midgut epithelium may inhibit the absorption of nutrients and stimulate endocytosis, which can lead to the disruption of the midgut cells.
The insect peritrophic matrix is composed of chitin, proteoglycans, and proteins. It serves as both a barrier to protect the midgut epithelium from abrasive food particles and as a semipermeable membrane to regulate passage of molecules between different midgut compartments (39). Binding of lectins such as GSII to this membrane may decrease its permeability and may affect the movement of molecules between the endo- and exoperitrophic spaces, thereby hindering the absorption of nutrients. Interference of GSII with digestive enzymes and assimilatory proteins causing inhibited food digestion and absorption may also contribute to the overall detrimental effect of GSII on nutrient absorption.
Despite differences in carbohydrate specificity in legume lectins, crystallographic analyses indicate that the carbohydrate-binding sites are very similar among legume lectins. We determined, by site-directed mutagenesis, that Asp88, Tyr134, and Asn136 of rGSII are essential for GlcNAc binding (33). Asp88, invariant in all known legume lectin sequences (1), normally forms a hydrogen bond with the carbohydrate substrate. When the carboxylated side chain (COO−) of Asp88 was replaced by CONH2 (D88N), it could no longer form hydrogen bonds. As a result, binding activity was diminished. Tyr134 is less conserved in legume lectins. However, these lectins all contain aromatic or bulky side chains that are essential for extensive van der Waals contact with the carbohydrate (1, 4). As expected, mutation Y134F, which preserves the aromatic side chain, did not attenuate GlcNAc binding or insecticidal activity. However, replacement of the phenolic side chain of Tyr134 with Asp or Gly eliminated carbohydrate-binding and biological activity. Asn136 is also an invariant residue in all legume lectin sequences known to date; therefore, as predicted, replacement with Gln rendered the protein biologically and biochemically inactive. Interestingly, the mutation N136D lowered, but did not abolish, GlcNAc binding at or near the physiological pH of the cowpea bruchid midgut, and this protein retained moderate insecticidal activity. Carbohydrate binding did not occur with this rGSII variant at pH 7.0 and 8.8. The Asn136 amide group (NH2) side chain hydrogen bonds with carbohydrate (37). When the CH2CONH2 side chain was replaced by CH2COOH (N136D), the GlcNAc-binding and insecticidal activities were dependent on whether the carboxyl group was ionized (pKa 3.9). At neutral or basic pH, the carboxyl group is ionized, preventing H-bond interaction with the carbohydrate substrate. However, as pH becomes acidic, the COO− groups gradually become protonated and available for H-bonding. Protonation restores GlcNAc binding, at least in part. Extension of the CH2CONH2 group to (CH2)2CONH2 (N136Q) altered the distance or angle for interaction with Ca2+ and the GlcNAc substrate and abolished the activity of the protein.
Our experiments show that the GlcNAc-binding rGSII and its mutant forms are more stable to cowpea bruchid gut proteolytic activity in vitro than are the nonbinding forms. Furthermore, these rGSII proteins survived passage through the gut in vivo and were recovered from the excreta in a form that retained GlcNAc-binding activity. Undoubtedly, plant defensive proteins that are protease resistant would be more effective than those that are not. The stability of GSII to proteolysis could be the result of a stable tertiary structure inherent to it and all variants with GlcNAc-binding and insecticidal activities. Alternatively, binding to glycoconjugates in the gut may somehow protect the lectins from proteolytic activity, therefore stabilizing them. Because most lectins are at least partially heat stable and survive passage through the gastrointestinal tract, including some nontoxic lectins (17, 40), differential survival of the lectins in the gut does not by itself explain their toxicity. However, biochemical stability is prerequisite for the biological activity of the protein.
The insecticidal activity of lectins make them good candidates for the control of insect pests. However, the specific interaction of lectins with the gut epithelium of insects may have a wider potential for biotechnology than the insecticidal property of lectins alone. By developing chimeric proteins that use lectins as a binding domain or subunit, they might be used, (i) to assist in concentrating bioactive polypeptides such as Bt toxins and insect hormones at or near vulnerable target sites in the insect alimentary tract, (ii) to facilitate the transfer of cytotoxins across the epithelial cell membrane or (iii) to help prevent proteolytic digestion of other bioactive proteins to which they have been bound.
Acknowledgments
This is publication No. 15826, Purdue University Agricultural Experimental Station, West Lafayette, IN. This research was supported by a grant from National Research Initiative, United States Department of Agriculture Competitive Grant, Award No. 95-37302-1804.
ABBREVIATIONS
- GSII
Griffonia simplicifolia lectin II
- rGSII
recombinant large subunit of GSII
- GlcNAc
N-acetylglucosamine
- PHA
bean phytohemagglutinin
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Sharon N, Lis H. FASEB J. 1990;4:3198–3208. doi: 10.1096/fasebj.4.14.2227211. [DOI] [PubMed] [Google Scholar]
- 2.Raikhel N V, Lee H I, Broekaert W F. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:591–615. [Google Scholar]
- 3.Sharon N. Trends Biochem Sci. 1993;18:221–226. doi: 10.1016/0968-0004(93)90193-q. [DOI] [PubMed] [Google Scholar]
- 4.Weis W I, Drickamer K. Annu Rev Biochem. 1996;65:411–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 5.Diaz C L, van Spronsen P C, Bakhuizen R, Logman G J J, Lugtenberg E J J, Kijne J W. Planta. 1986;168:350–359. doi: 10.1007/BF00392360. [DOI] [PubMed] [Google Scholar]
- 6.Bauchrowitz M A, Barker D G, Truchet G. Plant J. 1996;9:31–43. [Google Scholar]
- 7.Diaz C L, Melchers L S, Hooykaas P J J, Lugtenberg B J J, Kijne J W. Nature (London) 1989;338:579–581. [Google Scholar]
- 8.van Eijsden R R, Diaz C L, de Pater B S, Kijne J W. Plant Mol Biol. 1995;29:431–439. doi: 10.1007/BF00020975. [DOI] [PubMed] [Google Scholar]
- 9.Peumans W J, Van Damme E J M. Plant Physiol. 1995;109:347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitches E, Gatehouse A M R, Gatehouse J A. J Insect Physiol. 1997;43:727–739. doi: 10.1016/s0022-1910(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 11.Rahbe Y, Sauvion N, Febvay G, Peumans W J, Gatehouse A M R. Entomol Exp Appl. 1995;76:143–155. [Google Scholar]
- 12.Czapla T H, Lang B A. J Econ Entomol. 1990;83:2480–2485. [Google Scholar]
- 13.Murdock L L, Huesing J E, Nielsen S S, Pratt R C, Shade R E. Phytochemistry. 1990;29:85–89. [Google Scholar]
- 14.Habibi J, Backus E A, Czapla T H. J Econ Entomol. 1993;86:945–951. [Google Scholar]
- 15.Zhu K, Huesing J E, Shade R E, Bressan R A, Hasegawa P M, Murdock L L. Plant Physiol. 1996;110:195–202. doi: 10.1104/pp.110.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pusztai A. In: Plant Lectins. Phillipson J D, Ayres D C, Baxter H, editors. Cambridge, U.K.: Cambridge Univ. Press; 1991. pp. 105–205. [Google Scholar]
- 17.Pusztai A, Greer F, Grant G. Biochem Society Trans. 1989;17:481–482. [Google Scholar]
- 18.Pusztai A, Koninkx J, Hendriks H, Kok W, Hulscher S, Van Damme E J M, Peumans W J, Grant G, Bardocz S. J Nutr Biochem. 1996;7:677–682. [Google Scholar]
- 19.Kim Y S, Brophy E J, Nicholson J A. J Biol Chem. 1976;251:3206–3212. [PubMed] [Google Scholar]
- 20.Hisayasu S, Orimo H, Migita S, Ikeda Y, Satoh K, Shinjo S, Hirai Y, Yoshino Y. J Nutr. 1992;122:1190–1196. doi: 10.1093/jn/122.5.1190. [DOI] [PubMed] [Google Scholar]
- 21.Santiago J G, Levy-Benshimol A, Carmona A. J Nutr Biochem. 1993;4:426–430. [Google Scholar]
- 22.Janssen O, Scheffler A, Kabelitz D. Arzneim-Forsch. 1993;43:1221–1227. [PubMed] [Google Scholar]
- 23.Kim M, Rao M V, Tweardy D J, Prakash M, Galili U, Gorelik E. Glycobiology. 1993;3:447–453. doi: 10.1093/glycob/3.5.447. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni G V, McCulloch C A G. J Cell Physiol. 1995;165:119–133. doi: 10.1002/jcp.1041650115. [DOI] [PubMed] [Google Scholar]
- 25.Leist M, Wendel A. J Hepatol. 1996;25:948–959. doi: 10.1016/s0168-8278(96)80301-1. [DOI] [PubMed] [Google Scholar]
- 26.Chrispeels M J, Raikhel N V. Plant Cell. 1991;3:1–9. doi: 10.1105/tpc.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisemann C H, Donaldson R A, Pearson R D, Cadogan L C, Vuocolo T, Tellam R L. Entomol Exp Appl. 1994;72:1–10. [Google Scholar]
- 28.Gatehouse A M R, Shackley S J, Fenton K A, Bryden J, Pusztai A. J Sci Food Agric. 1989;47:269–280. [Google Scholar]
- 29.Huesing J E, Shade R E, Chrispeels M J, Murdock L L. Plant Physiol. 1991;96:993–996. doi: 10.1104/pp.96.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harper S M, Crenshaw R W, Mullins M A, Privalle L S. J Econ Entomol. 1995;88:1197–1202. [Google Scholar]
- 31.Ebisu S, Iyer P N S, Goldstein I J. Carbohydr Res. 1978;61:129–138. [Google Scholar]
- 32.Zhu-Salzman, K., Salzman, R. A., Koiwa, H., Murdock, L. L., Bressan, R. A. & Hasegawa, P. M. (1998) Physiol. Plant., in press.
- 33.Zhu K, Bressan R A, Hasegawa P M, Murdock L L. FEBS Lett. 1996;390:271–274. doi: 10.1016/0014-5793(96)00671-0. [DOI] [PubMed] [Google Scholar]
- 34.Kitch L W, Murdock L L. Arch Insect Biochem Physiol. 1986;3:561–575. [Google Scholar]
- 35.Shade R E, Murdock L L, Foard D E, Pomeroy M A. Environ Entomol. 1986;15:1286–1291. [Google Scholar]
- 36.Shade, R. E., Furgason, E. S. & Murdock, L. L. (1990) Am. Entomologist 231–234.
- 37.Delbaere L T J, Vandonselaar M, Prasad L, Quail J W, Wilson K S, Dauter Z. J Mol Biol. 1993;230:950–965. doi: 10.1006/jmbi.1993.1212. [DOI] [PubMed] [Google Scholar]
- 38.Gatehouse A M R, Butler K J, Fenton K A, Gatehouse J A. Entomol Exp Appl. 1985;39:279–286. [Google Scholar]
- 39.Lehane M J. Annu Rev Entomol. 1997;42:525–550. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- 40.Herzig K H, Bardocz S, Grant G, Nustede R, Folsch U R, Pusztai A. Gut. 1997;41:333–338. doi: 10.1136/gut.41.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]