Abstract
Evidence is reported that shows the presence in Escherichia coli K-12 of a newly found acetolactate synthase. This enzyme is the product of two genes, ilvH and ilvI, both located very close to leu. Amber mutations have been found in both genes and therefore their products are polypeptides. Mutations in the ilvH gene cause the appearance of an acetolactate synthase activity which is relatively resistant to valine inhibition and can be separated by adsorption on hydroxylapatite from another activity present in the extract and more sensitive to valine inhibition than the former. A mutant altered in the ilvI gene was isolated among the revertants sensitive to valine inhibition of an ilvH mutant. Such a mutant lacks the resistant acetolactate synthase. A temperature-sensitive revertant of the ilvI mutant contained a temperature-sensitive acetolactate synthase. Thus ilvI is the structural gene for a specific acetolactate synthase. The activity of the ilvH gene product has been measured by adding an extract containing it to a purified ilvI acetolactate synthase, which, upon incubation, became more sensitive to valine inhibition. Conversely, a valine-sensitive acetolactate synthase (the product of the ilvH and the ilvI genes) became more resistant to valine inhibition upon incubation with an extract of a strain containing a missense ilvH gene product.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh T., Ozeki H. Suppressor gene Su3+ of E. coli, a structural gene for tyrosine TRNA. Proc Natl Acad Sci U S A. 1968 Mar;59(3):792–799. doi: 10.1073/pnas.59.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt J. M., Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XX. Multiple forms of acetohydroxy acid synthetase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):444–450. doi: 10.1016/s0006-291x(72)80071-8. [DOI] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
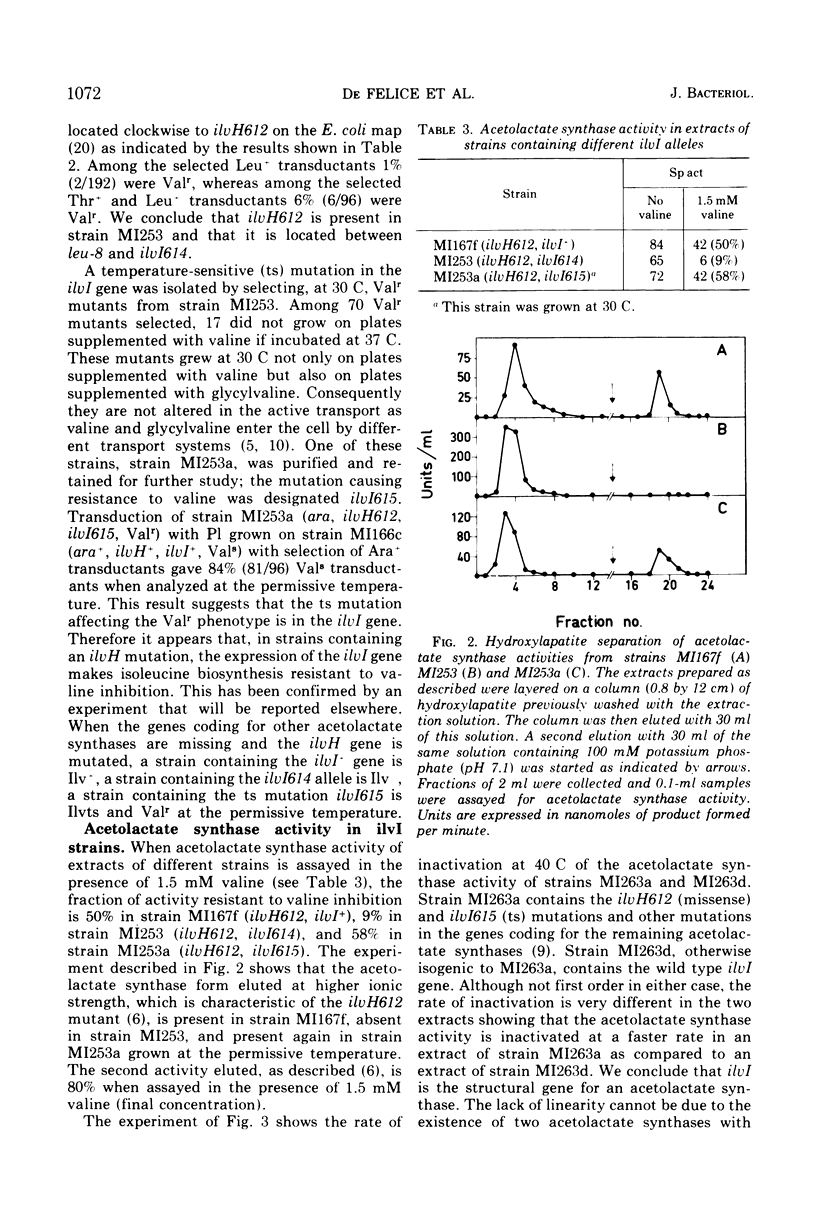
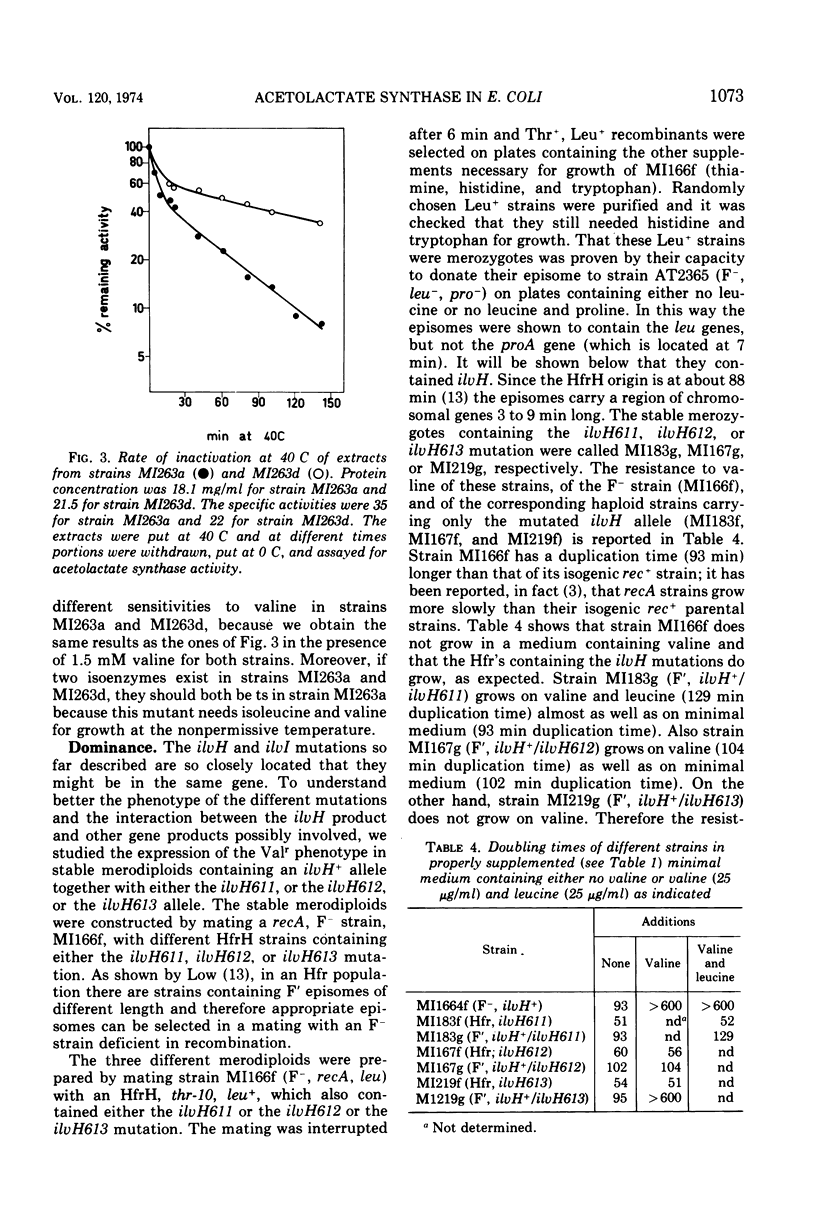
- De Felice M., Guardiola J., Lamberti A., Iaccarino M. Escherichia coli K-12 mutants altered in the transport systems for oligo- and dipeptides. J Bacteriol. 1973 Nov;116(2):751–756. doi: 10.1128/jb.116.2.751-756.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Malorni M. C., Klopotowski T., Iaccarino M. Regulation of the pool size of valine in Escherichia coli K-12. J Bacteriol. 1974 Dec;120(3):1058–1067. doi: 10.1128/jb.120.3.1058-1067.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Iaccarino M. Mutant of Escherichia coli K-12 missing acetolactate synthase activity. J Bacteriol. 1974 Oct;120(1):536–538. doi: 10.1128/jb.120.1.536-538.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Mutations affecting the different transport systems for isoleucine, leucine, and valine in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):393–405. doi: 10.1128/jb.117.2.393-405.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Isoleucine auxotrophy as a consequence of a mutationally altered isoleucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Feb;105(2):527–537. doi: 10.1128/jb.105.2.527-537.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritani K, Matsuno T, Ikeda Y. Genetic and Biochemical Studies on Isoleucine and Valine Requiring Mutants of ESCHERICHIA COLI. Genetics. 1965 Mar;51(3):341–349. doi: 10.1093/genetics/51.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Temperature-sensitive growth inhibition by valine in Salmonella typhimurium: alteration of one form of acetohydroxy acid synthetase. J Bacteriol. 1973 Oct;116(1):98–106. doi: 10.1128/jb.116.1.98-106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Two forms of biosynthetic acetohydroxy acid synthetase in Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Jul 25;48(2):437–443. doi: 10.1016/s0006-291x(72)80070-6. [DOI] [PubMed] [Google Scholar]
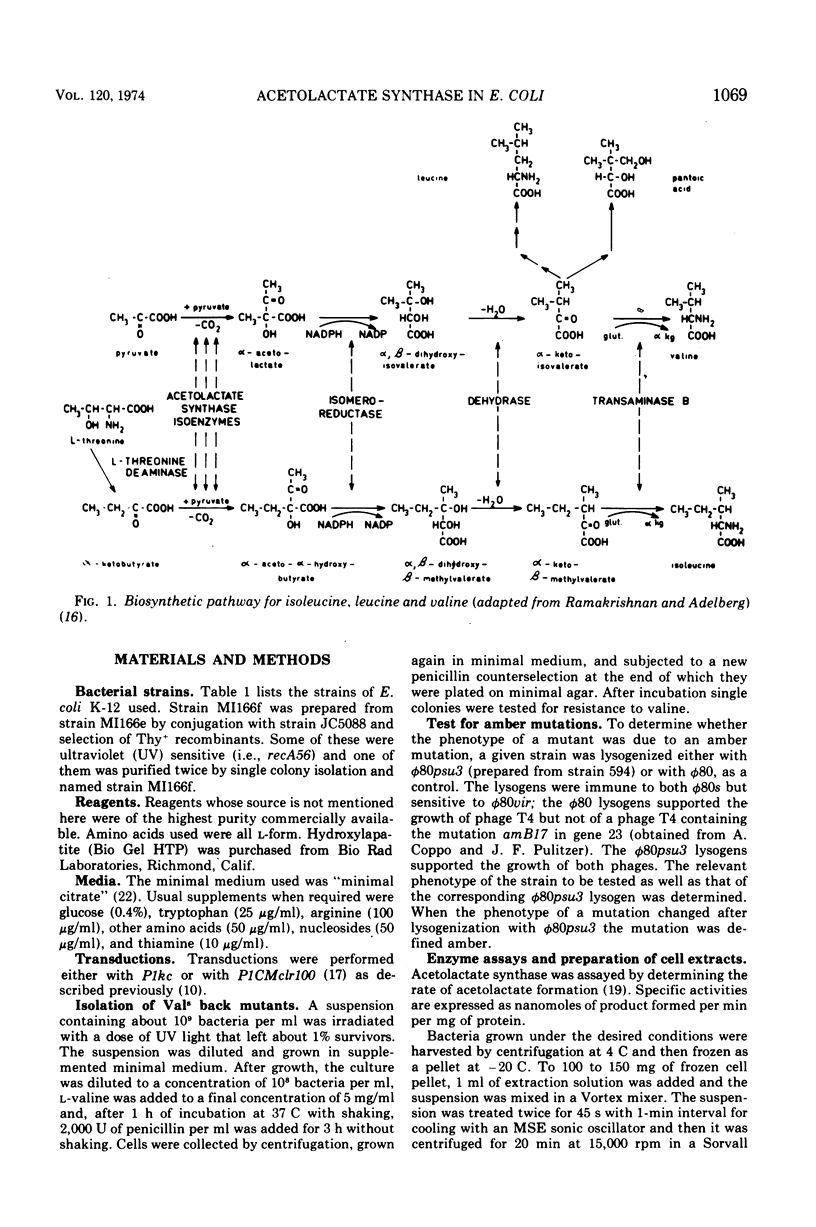
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. V. Antagonism between isoleucine and valine. J Bacteriol. 1955 Aug;70(2):241–248. doi: 10.1128/jb.70.2.241-248.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


