Abstract
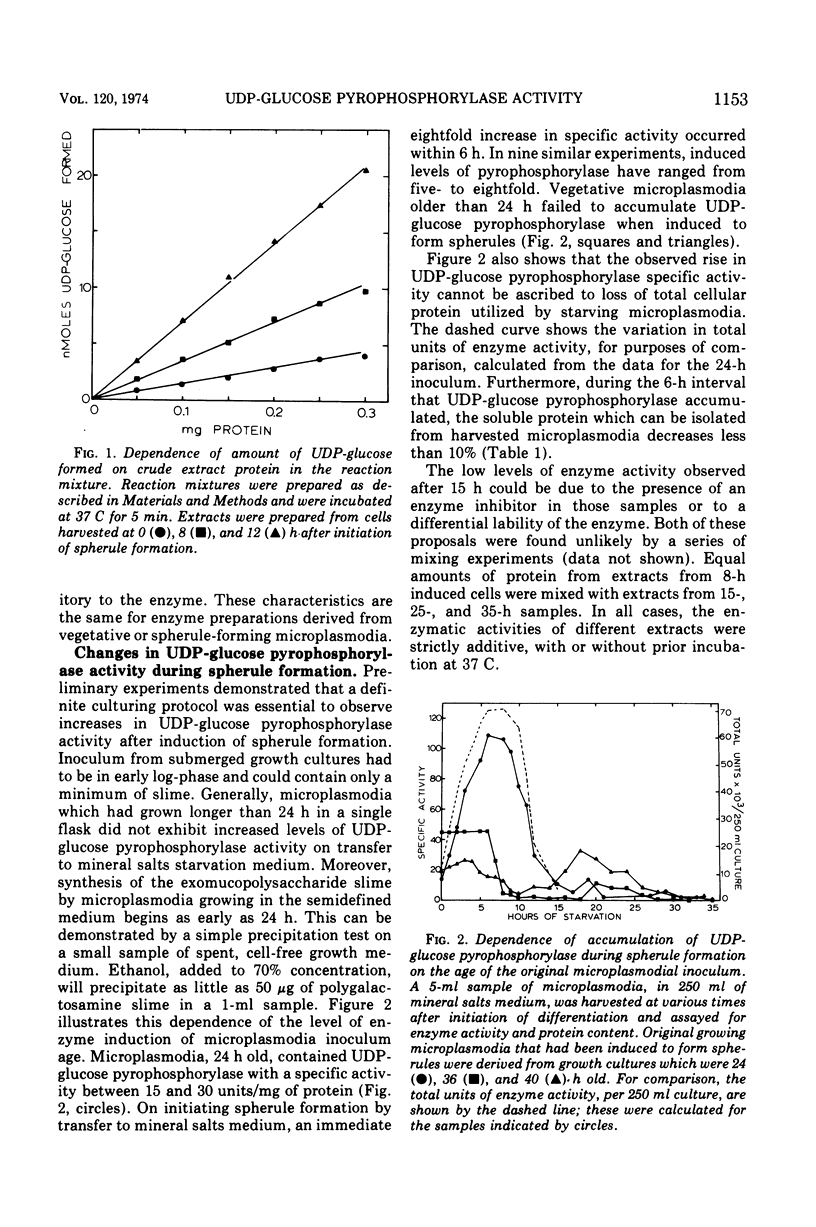
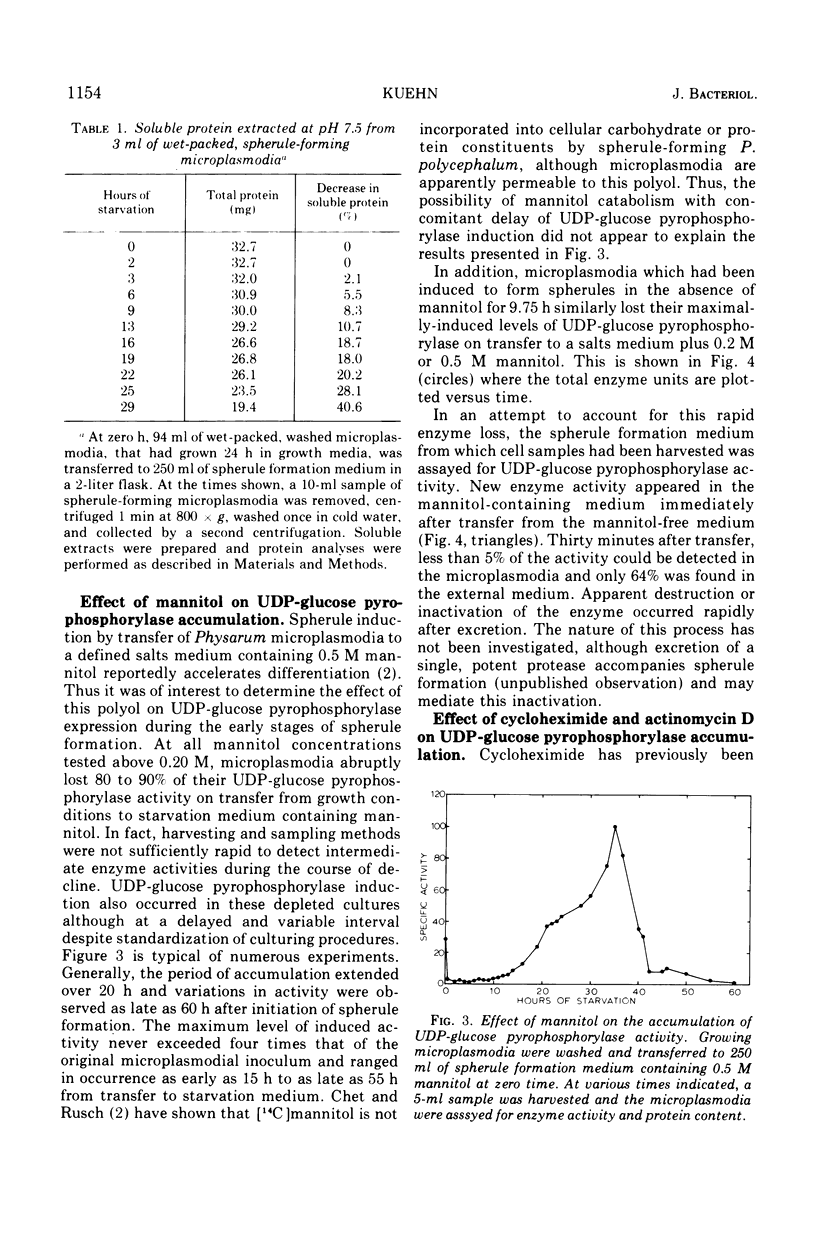
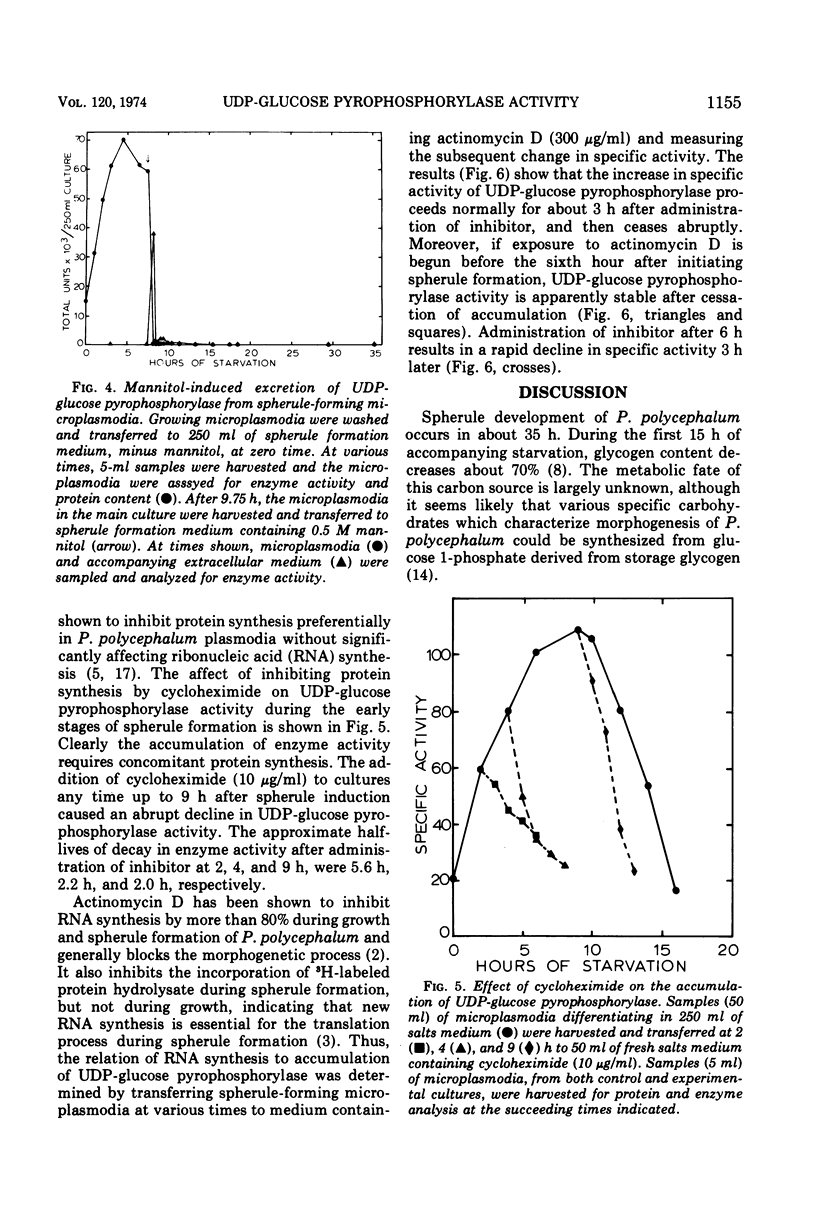
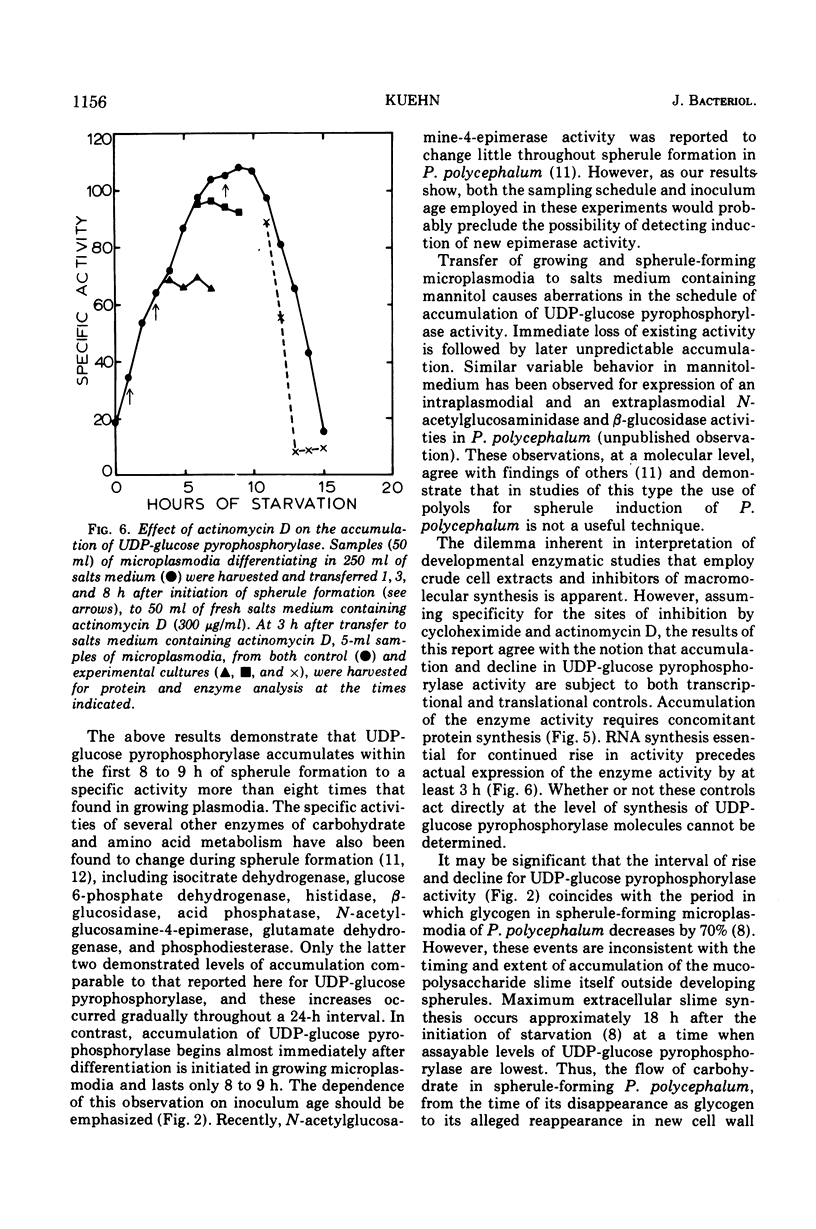
The specific activity of uridine 5′-triphosphate:α-d-glucose 1-phosphate uridyltransferase (EC 2.7.7.9) (also called uridine 5′-diphosphate [UDP]-glucose pyrophosphorylase) has been found to increase up to eightfold during spherule formation by the slime mold Physarum polycephalum. The enzyme accumulates during the first 8 to 9 h after initiation of spherule formation, declines to basal levels found in vegetative microplasmodia by 15 h, and is undetectable in completed spherules. Specific activities for UDP-glucose pyrophosphorylase in vegetative microplasmodia range from 15 to 30 nmol of UDP-glucose formed per min per mg of protein, whereas accumulated levels during spherule formation can attain a specific activity as high as 125 nmol of UDP-glucose formed per min per mg of protein. The scheduling and extent of accumulation are critically dependent on an early log-phase age of microplasmodia originally induced to form spherules. Spherule induction by 0.2 M or 0.5 M mannitol delays this schedule in a variable and unpredictable manner. Spherule-forming microplasmodia which have accumulated high levels of UDP-glucose pyrophosphorylase spontaneously excrete the enzyme when transferred to salts medium containing 0.2 M or 0.5 M mannitol. The excreted enzyme is subsequently destroyed or inactivated. Studies with preferential inhibitors of macromolecular synthesis indicate that accumulation of UDP-glucose pyrophosphorylase requires concomitant protein synthesis and prior ribonucleic acid synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Sussman M. The appearance and disappearance of uridine diphosphate glucose pyrophosphorylase activity during differntiation of the cellular slime mold Dictyostelium discoideum. J Biol Chem. 1967 Apr 25;242(8):1696–1700. [PubMed] [Google Scholar]
- Chet I., Rusch H. P. Induction of spherule formation in Physarum polycephalum by polyols. J Bacteriol. 1969 Nov;100(2):673–678. doi: 10.1128/jb.100.2.673-678.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chet I., Rusch H. P. RNA differences between spherulating and growing microplasmodia of Physarum polycephalum as revealed by sedimentation pattern and DNA-RNA hybridization. Biochim Biophys Acta. 1970;209(2):559–568. doi: 10.1016/0005-2787(70)90753-7. [DOI] [PubMed] [Google Scholar]
- Chin B., Bernstein I. A. Adenosine triphosphate and synchronous mitosis in Physarum polycephalum. J Bacteriol. 1968 Aug;96(2):330–337. doi: 10.1128/jb.96.2.330-337.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins J. E., Brewer E. N., Rusch H. P. The effect of actidione on mitosis in the slime mold Physarum polycephalum. J Cell Biol. 1965 Nov;27(2):337–341. doi: 10.1083/jcb.27.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmundson T. D., Ashworth J. M. 6-phosphogluconate dehydrogenase and the assay of uridine diphosphate glucose pyrophosphorylase in the cellular slime mould Dictyostelium discoideum. Biochem J. 1972 Feb;126(3):593–600. doi: 10.1042/bj1260593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke J., Sussman M. Accumulation of uridine diphosphoglucose pyrophosphorylase in Dictyostelium discoideum via preferential synthesis. J Mol Biol. 1973 Dec 5;81(2):173–185. doi: 10.1016/0022-2836(73)90187-3. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Rusch H. P. Ultrastructural changes during spherule formation in Physarum polycephalum. J Ultrastruct Res. 1970 Jan;30(1):172–183. doi: 10.1016/s0022-5320(70)90071-7. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Sauer H. W., Sauer L., Rusch H. P. Polyphosphate and other phosphorus compounds during growth and differentiation of Physarum polycephalum. Can J Microbiol. 1969 Nov;15(11):1325–1331. doi: 10.1139/m69-240. [DOI] [PubMed] [Google Scholar]
- Gustafson G. L., Kong W. Y., Wright B. E. Analysis of uridine diphosphate-glucose pyrophosphorylase synthesis during differentiation in Dictyostelium discoideum. J Biol Chem. 1973 Jul 25;248(14):5188–5196. [PubMed] [Google Scholar]
- Hiatt W. R., Whiteley H. R. Activity of uridine diphosphate N-acetylglucosamine-4-epimerase during spherulation of Physarum polycephalum. J Bacteriol. 1974 May;118(2):761–763. doi: 10.1128/jb.118.2.761-763.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCormick J. J., Blomquist J. C., Rusch H. P. Isolation and Characterization of a Galactosamine Wall from Spores and Spherules of Physarum polycephalum. J Bacteriol. 1970 Dec;104(3):1119–1125. doi: 10.1128/jb.104.3.1119-1125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. C., Sussman M. Uridine diphosphate glucose pyrophosphorylase in Dictyostelium discoideum. Stability and developmental fate. J Biol Chem. 1969 Jun 10;244(11):2990–2995. [PubMed] [Google Scholar]
- Pannbacker R. G. Uridine diphosphoglucose biosynthesis during differentiation in the cellular slime mold. I. In vivo measurements. Biochemistry. 1967 May;6(5):1283–1286. doi: 10.1021/bi00857a008. [DOI] [PubMed] [Google Scholar]
- SHEN L., PREISS J. BIOSYNTHESIS OF BACTERIAL GLYCOGEN. I. PURIFICATION AND PROPERTIES OF THE ADENOSINE DIPHOSPHOGLUCOSE PYROPHOSPHORYLASE OF ARTHROBACTER SPECIES NRRL B1973. J Biol Chem. 1965 Jun;240:2334–2340. [PubMed] [Google Scholar]
- SUSSMAN M., OSBORN M. J. UDP-GALACTOSE POLYSACCHARIDE TRANSFERASE IN THE CELLULAR SLIME MOLD, DICTYOSTELIUM DISCOIDEUM: APPEARANCE AND DISAPPEARANCE OF ACTIVITY DURING CELL DIFFERENTIATION. Proc Natl Acad Sci U S A. 1964 Jul;52:81–87. doi: 10.1073/pnas.52.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H. W., Babcock K. L., Rusch H. P. Changes in RNA synthesis associated with differentiation (sporulation) in Physarum polycephalum. Biochim Biophys Acta. 1969 Dec 16;195(2):410–421. doi: 10.1016/0005-2787(69)90648-0. [DOI] [PubMed] [Google Scholar]
- WRIGHT B. E., ANDERSON M. L. Biochemical differentiation in the slime mold. Biochim Biophys Acta. 1959 Feb;31(2):310–322. doi: 10.1016/0006-3002(59)90003-4. [DOI] [PubMed] [Google Scholar]
- Weeks G., Ashworth J. M. Glycogen synthetase and the control of glycogen synthesis in the cellular slime mould Dictyostelium discoideum during the growth (myxamoebal) phase. Biochem J. 1972 Feb;126(3):617–626. doi: 10.1042/bj1260617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheals A. E. A homothallic strain of the myxomycete Physarum polycephalum. Genetics. 1970 Dec;66(4):623–633. doi: 10.1093/genetics/66.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. E., Dahlberg D. Stability in vitro of uridine diphosphoglucose pyrophosphorylase in Dictyostelium discoideum. J Bacteriol. 1968 Mar;95(3):983–985. doi: 10.1128/jb.95.3.983-985.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B., Simon W., Walsh B. T. A kinetic model of metabolism essential to differentiation in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1968 Jun;60(2):644–651. doi: 10.1073/pnas.60.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]


