Abstract
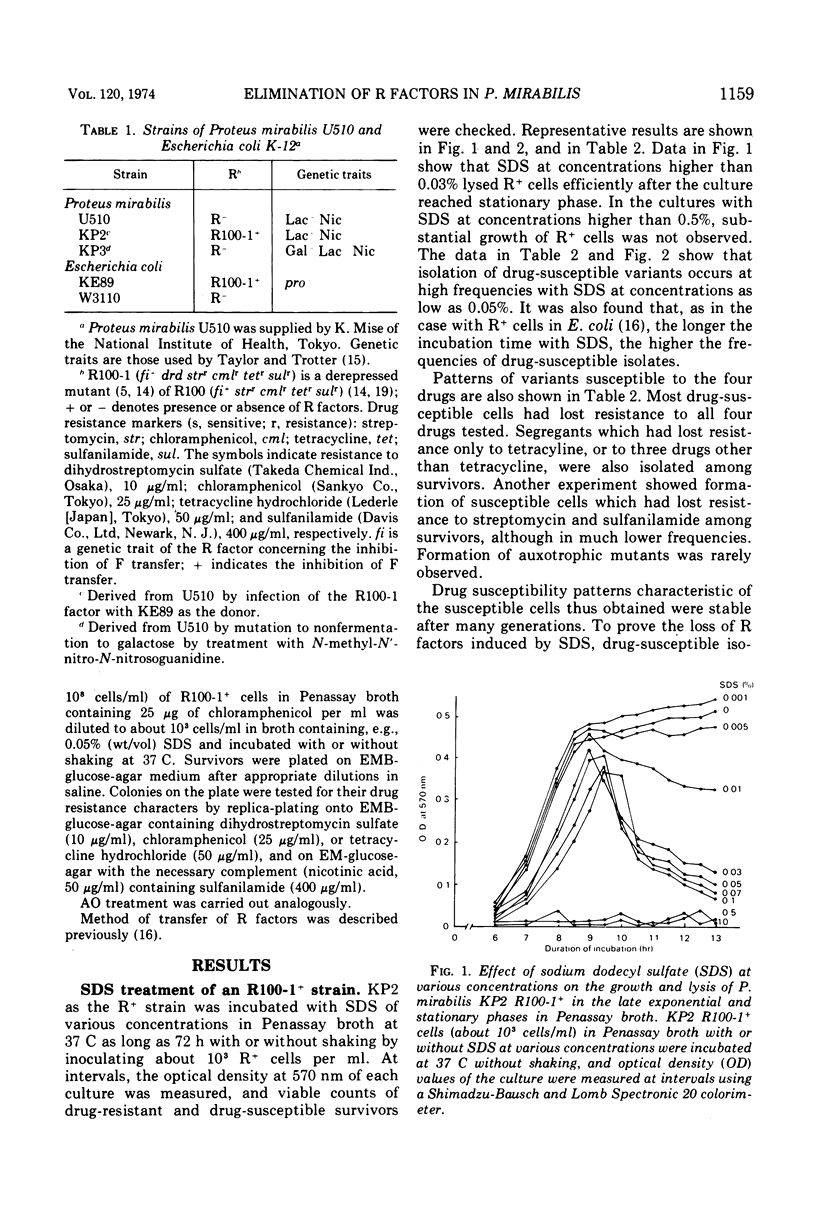
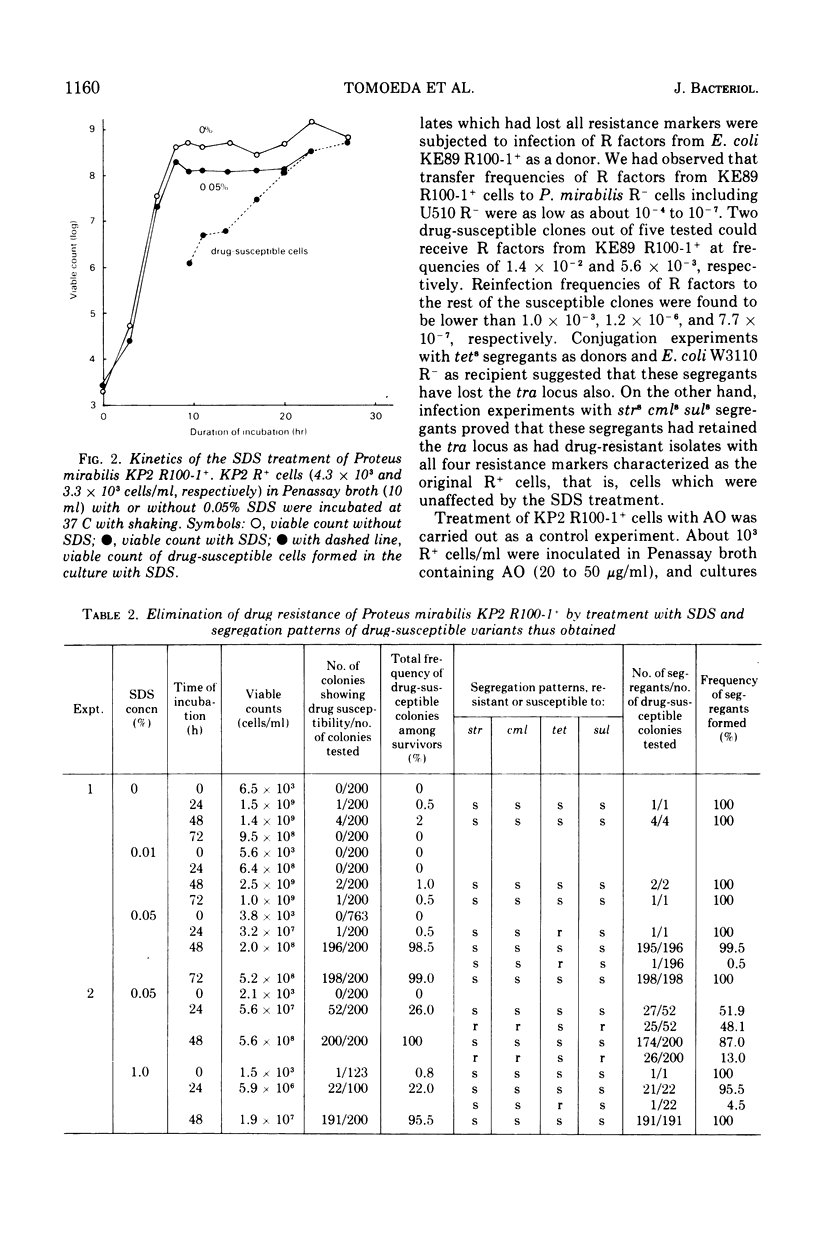
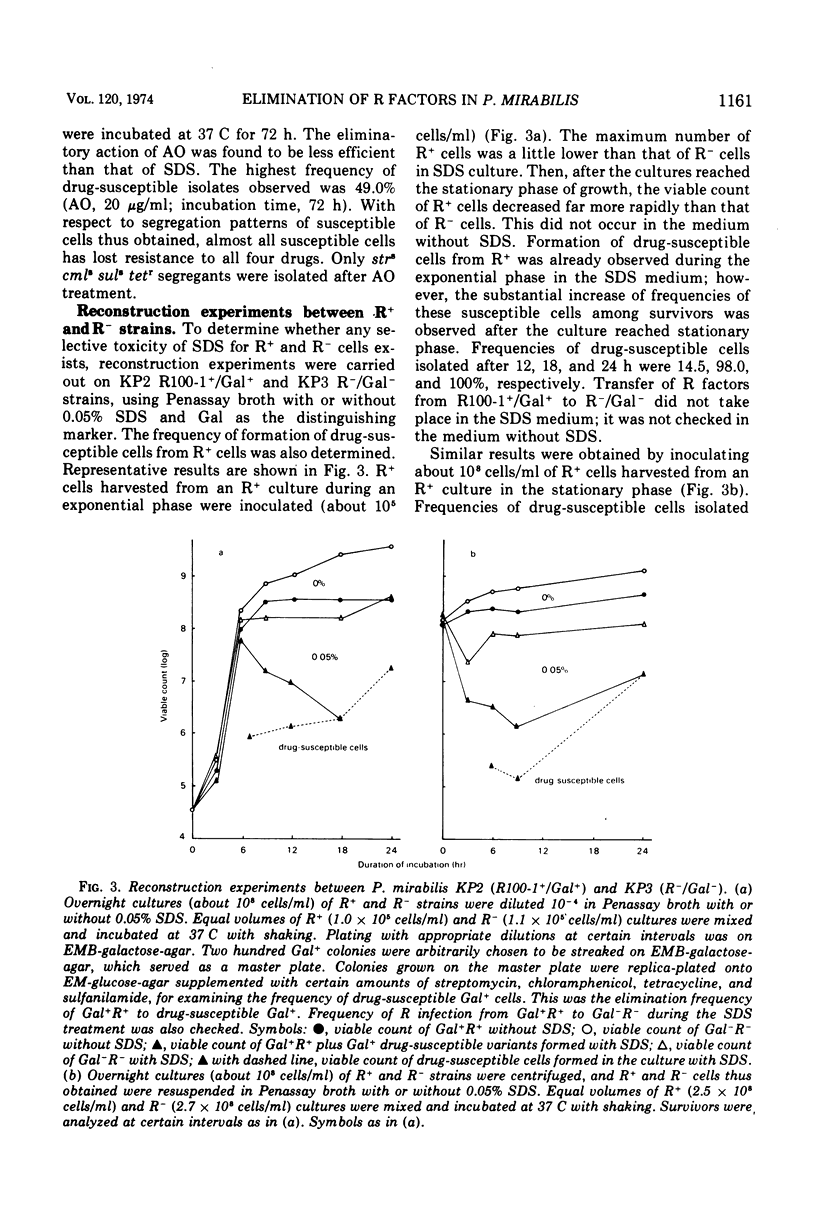
Growth of Proteus mirabilis harboring R100-1 (fi+drd strrcmlrtetrsulr) factors in Penassay broth containing sodium dodecyl sulfate (SDS) leads to the loss of all or part of the genetic elements in high frequencies. In media containing SDS at concentrations as low as 0.03%, both lysis of R+ cells and elimination of the R factors occur at high frequencies. Appearance of drug-susceptible cells in R+ cultures occurs during the exponential phase of growth; however, the frequencies of susceptible cells increase substantially after the culture reaches the stationary phase. Reconstruction experiments, coupled with other observations, suggest that the major factor in altering the frequency of drug-susceptible variants is the greater resistance of the variants to the lytic action of SDS. This resistance correlates in most cases with the loss of the transfer functions in the resistance transfer factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Nakano M., Inuzuka M., Tomoeda M. Specific role of sex pili in the effective eliminatory action of sodium dodecyl sulfate on sex and drug resistance factors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1114–1124. doi: 10.1128/jb.109.3.1114-1124.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- HARADA K., KAMEDA M., SUZUKI M., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. II. TRANSDUCTION OF TRANSMISSIBLE DRUG-RESISTANCE (R) FACTORS WITH PHAGE EPSILON. J Bacteriol. 1963 Dec;86:1332–1338. doi: 10.1128/jb.86.6.1332-1338.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Hirota Y. Gene recombination and segregation of resistance factor R in Escherichia coli. J Bacteriol. 1966 Jan;91(1):51–62. doi: 10.1128/jb.91.1.51-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Mitsuhashi S. Drug resistance of enteric bacteria. VII. Recombination of R factors with tetracycline-sensitive mutants. J Bacteriol. 1966 Nov;92(5):1351–1356. doi: 10.1128/jb.92.5.1351-1356.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka N., Nakamura S., Inuzuka M., Tomoeda M. Specific action of sodium dodecyl sulfate on the sex factor of Escherichia coli K-12 Hfr strains. J Bacteriol. 1969 Nov;100(2):827–835. doi: 10.1128/jb.100.2.827-835.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., HIROTA Y. Conjugal fertility associated with resistance factor R in Escherichia coli. J Bacteriol. 1962 Nov;84:902–910. doi: 10.1128/jb.84.5.902-910.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury V., Hedges R. W., Datta N. Two modes of "curing" transmissible bacterial plasmids. J Gen Microbiol. 1972 May;70(3):443–452. doi: 10.1099/00221287-70-3-443. [DOI] [PubMed] [Google Scholar]
- Sonstein S. A., Baldwin J. N. Loss of the penicillinase plasmid after treatment of Staphylococcus aureus with sodium dodecyl sulfate. J Bacteriol. 1972 Jan;109(1):262–265. doi: 10.1128/jb.109.1.262-265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. III. Transduotion of resistance factors. J Bacteriol. 1961 Aug;82:202–209. doi: 10.1128/jb.82.2.202-209.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T., TAKANO T. Conversion of male bacteria of Escherichia coli K12 to resistance to f phages by infection with the episome "resistance transfer factor". Virology. 1962 May;17:217–219. doi: 10.1016/0042-6822(62)90108-3. [DOI] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M. Screening method of agents against the R factor by the use of an Hfr made by integrative suppression with an R factor. Antimicrob Agents Chemother. 1974 Apr;5(4):362–365. doi: 10.1128/aac.5.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]



