Abstract
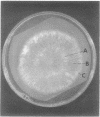
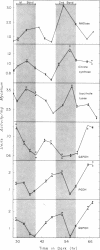
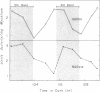
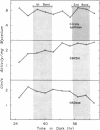
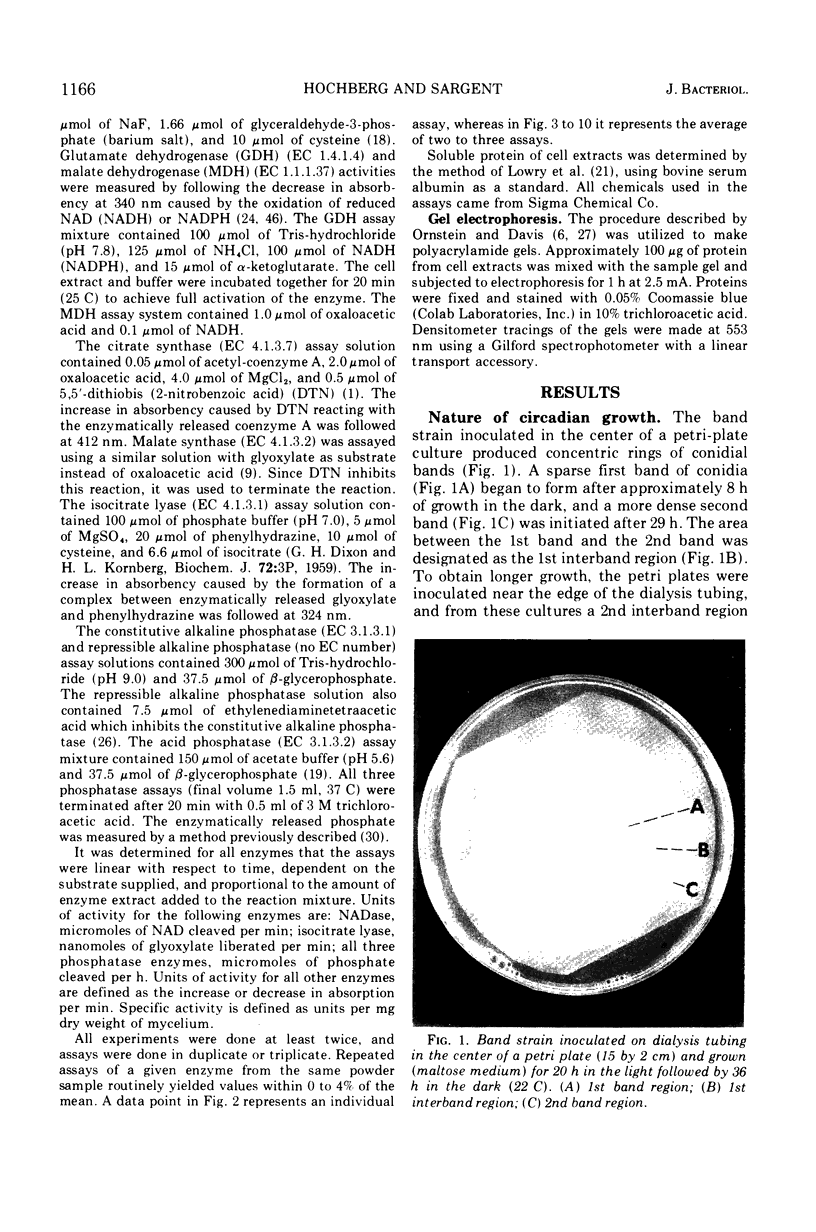
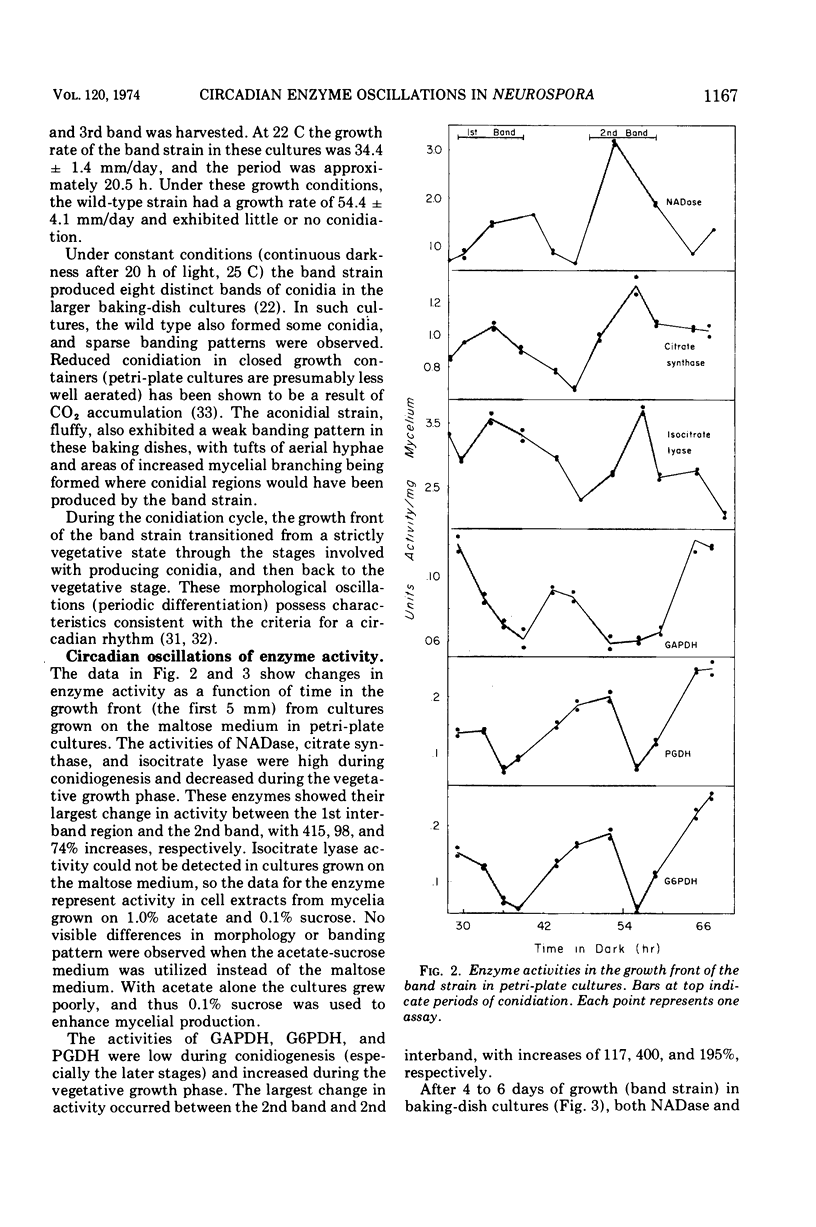
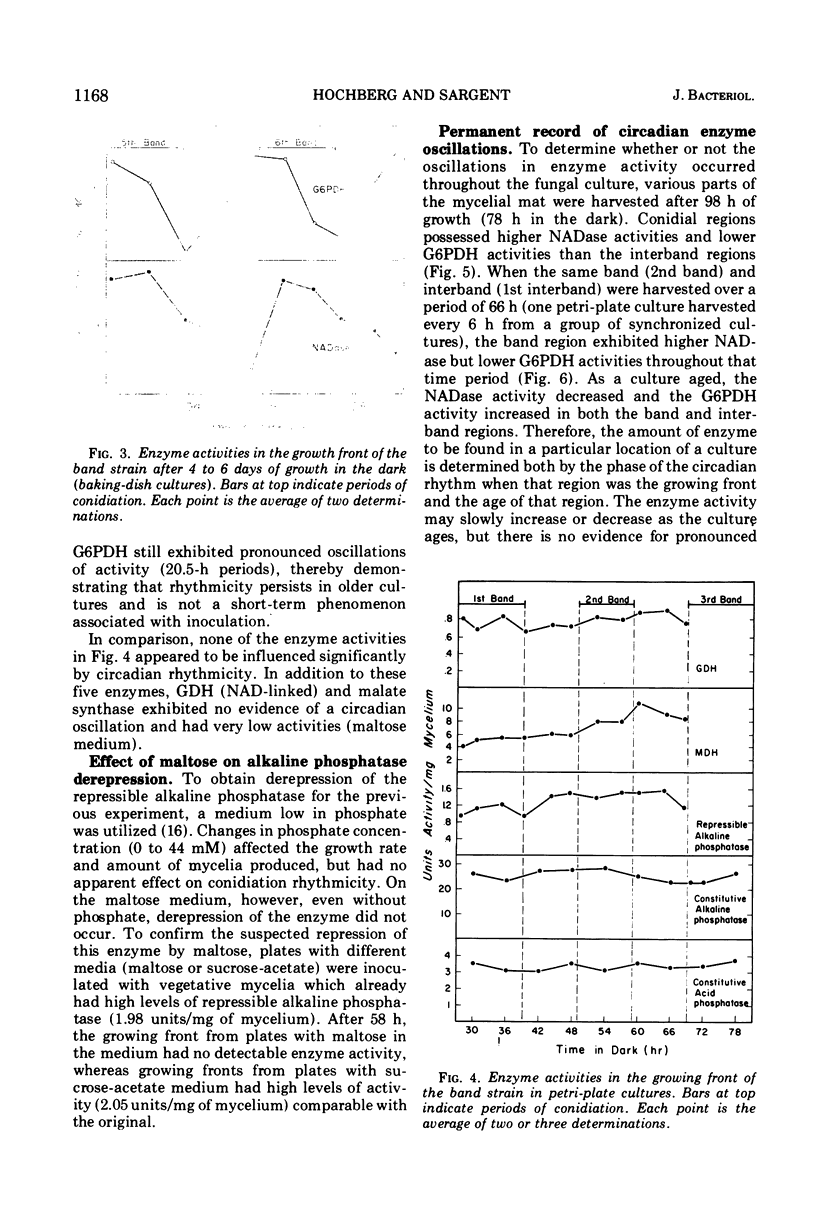
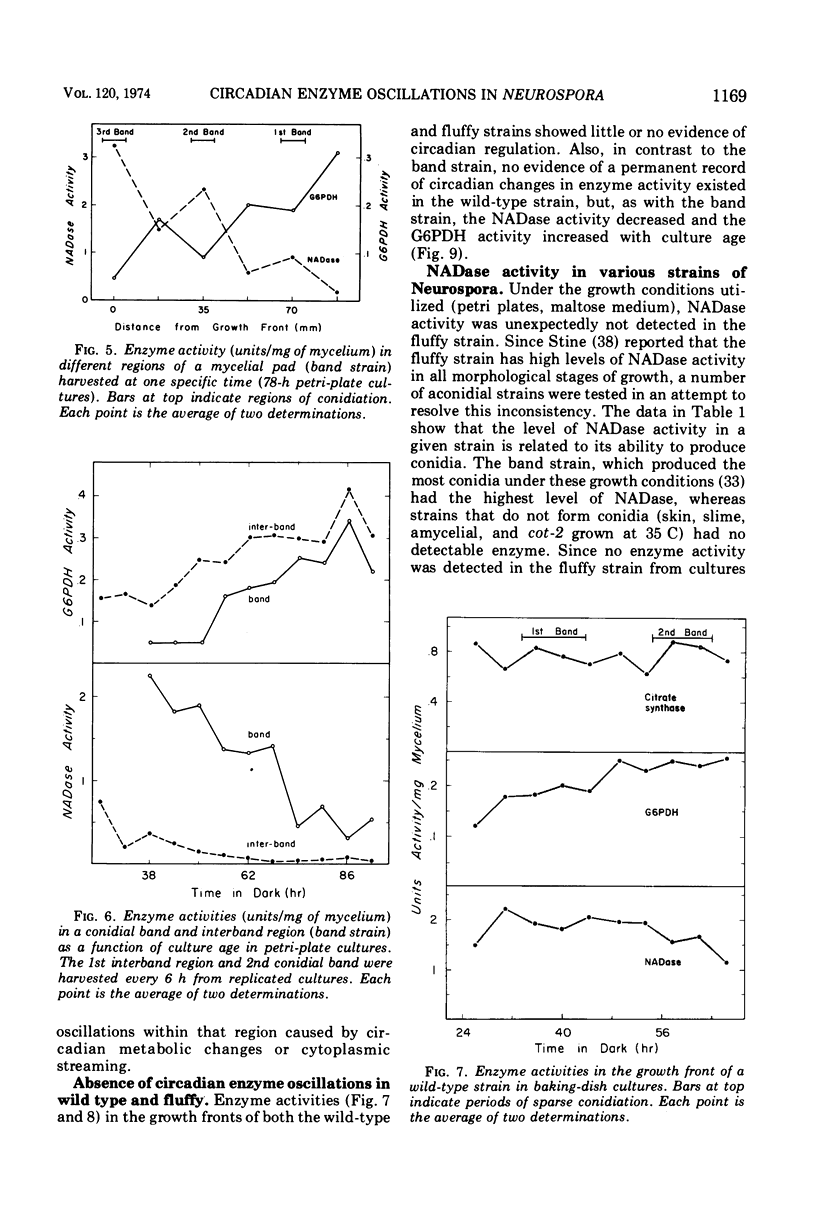
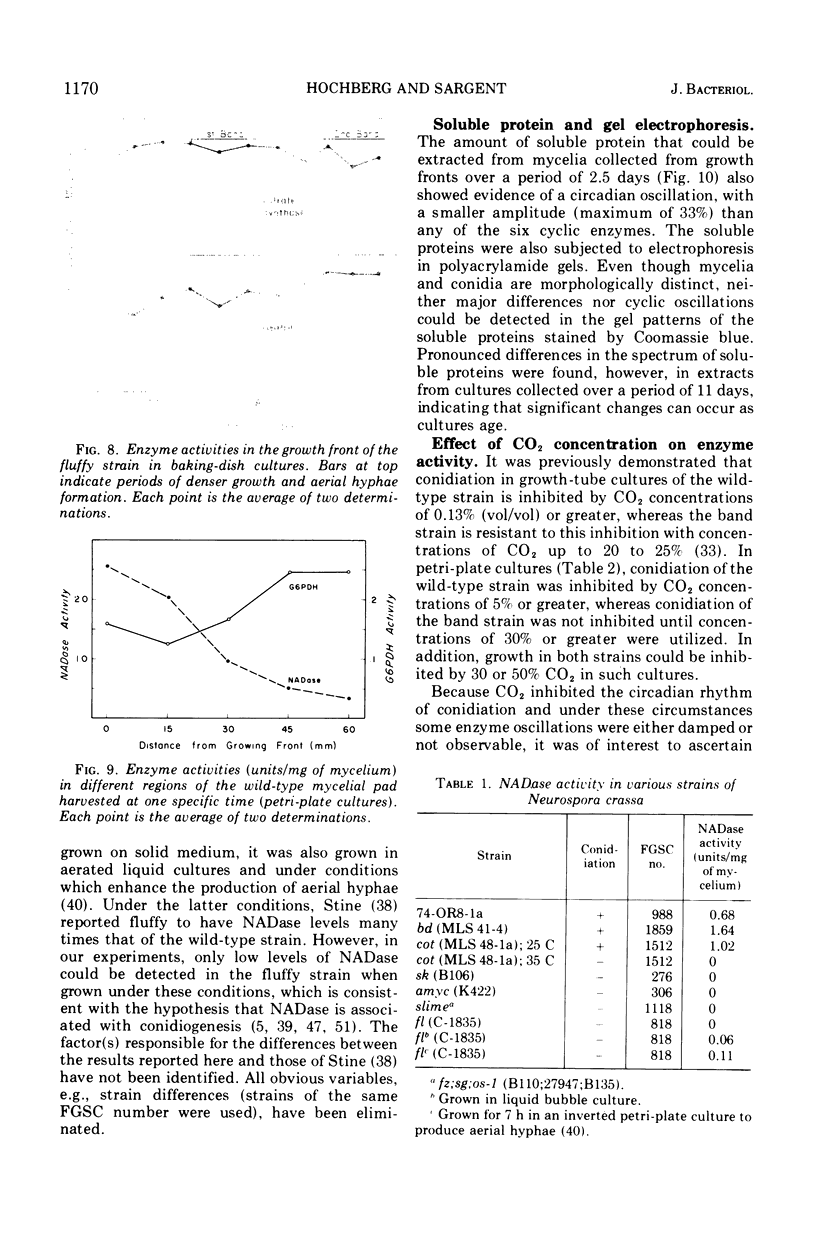
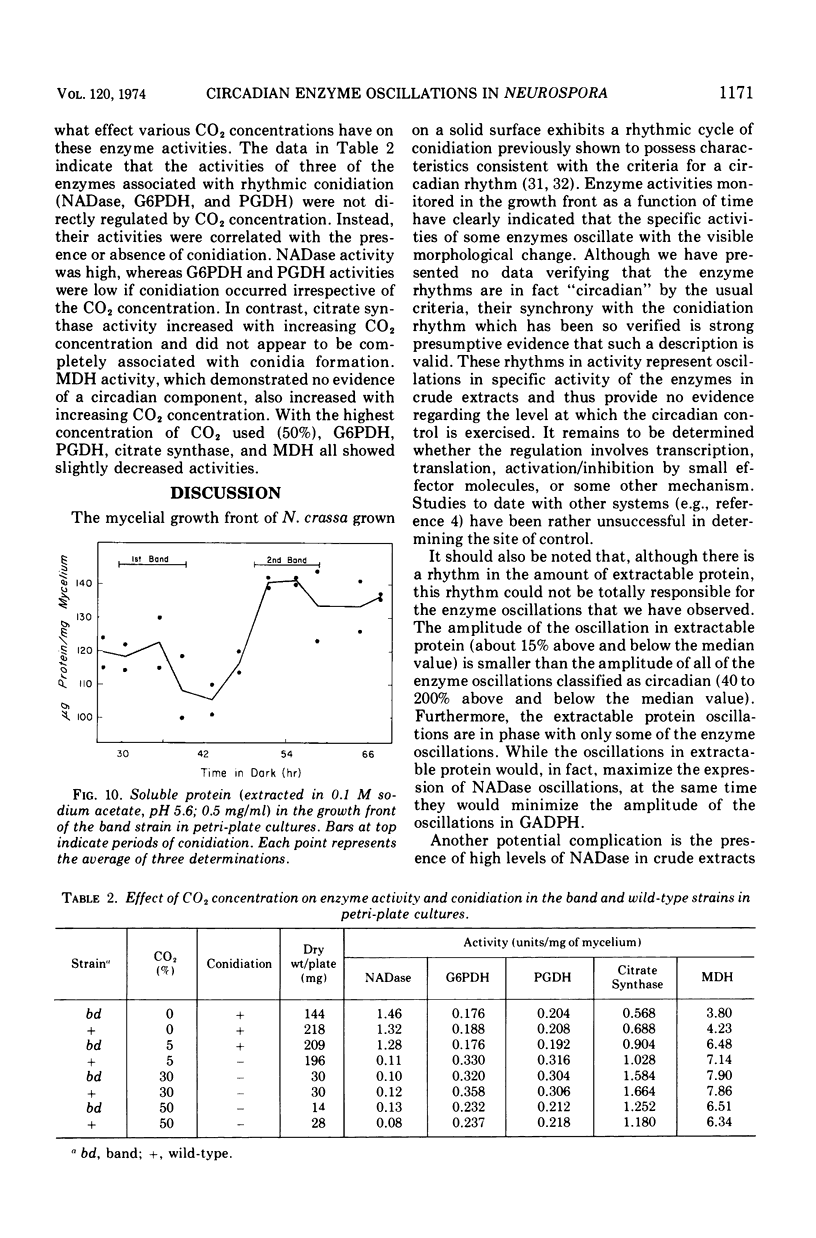
The mycelial growth front of the band strain of Neurospora grown on a solid surface exhibits a circadian rhythm of conidiation. Enzyme assays on extracts from that mycelium have shown that the activities of 6 of 13 enzymes (nicotinamide adenine dinucleotide nucleosidase, isocitrate lyase, citrate synthase, glyceraldehydephosphate dehydrogenase, phosphogluconate dehydrogenase, and glucose-6-phosphate dehydrogenase) and soluble-protein content oscillate with the visible morphological change. The rhythmic enzymes associated with the Krebs and glyoxylate cycles are more active during conidiogenesis, whereas the activities of the rhythmic enzymes of glycolysis and the hexose monophosphate shunt are reduced during that phase. The absence of enzyme oscillations in wild-type and fluffy strains which do not form conidia under the conditions employed suggests that the enzyme fluctuations are associated with conidiogenesis itself. Oscillations of enzyme activity as a function of time are restricted to the growth front. A permanent record of rhythmicity associated with conidial and nonconidial regions does, however, exist in the mycelial mat behind the growth front. The activities of three enzymes (nicotinamide adenine dinucleotide nucleosidase, glucose-6-phosphate dehydrogenase, and phosphogluconate dehydrogenase) are not directly influenced by CO2 concentration, but are correlated with the prescence or absence of conidiation which is controlled by CO2 concentration. In contrast, citrate synthase and malate dehydrogenase activities are correlated with changes in CO2 concentration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERS D. H., APPEL S. H., TOMKINS G. M. A SPECTROPHOTOMETRIC ASSAY FOR THIOGALACTOSIDE TRANSACETYLASE. J Biol Chem. 1965 Jan;240:10–13. [PubMed] [Google Scholar]
- Brody S., Harris S. Circadian rhythms in neurospora: spatial differences in pyridine nucleotide levels. Science. 1973 May 4;180(4085):498–500. doi: 10.1126/science.180.4085.498. [DOI] [PubMed] [Google Scholar]
- Brody S., Tatum E. L. The primary biochemical effect of a morphological mutation in Neurospora crassa. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1290–1297. doi: 10.1073/pnas.56.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. J., Sweeney B. M. The Activity of Ribulose Diphosphate Carboxylase in Extracts of Gonyaulax polyedra in the Day and the Night Phases of the Circadian Rhythm of Photosynthesis. Plant Physiol. 1972 Oct;50(4):446–451. doi: 10.1104/pp.50.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combépine G., Turian G. Activités de quelques enzymes associés à la conidiogenèse du Neurospora crassa. Arch Mikrobiol. 1970;72(1):36–47. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Feldman J. F., Hoyle M. N. Isolation of circadian clock mutants of Neurospora crassa. Genetics. 1973 Dec;75(4):605–613. doi: 10.1093/genetics/75.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-nonutilizing mutants of Neurospora rassa. II. Biochemical deficiencies and the roles of certain enzymes. J Bacteriol. 1968 Mar;95(3):1063–1068. doi: 10.1128/jb.95.3.1063-1068.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. The concurrent regulation of metabolically related enzymes. The Krebs cycle and glyoxylate shunt enzymes in Neurospora. Eur J Biochem. 1970 Dec;17(2):284–291. doi: 10.1111/j.1432-1033.1970.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. The regulation of synthesis of Krebs cycle enzymes in Neurospora by catabolite and end product repression. Eur J Biochem. 1970 Apr;13(3):548–553. doi: 10.1111/j.1432-1033.1970.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Fuscaldo K. E., Lechner J. F., Bazinet G. Genetic and biochemical studies of the hexose monophosphate shunt in Neurospora crassa. I. The influence of genetic defects in the pathway on colonial morphology. Can J Microbiol. 1971 Jun;17(6):783–788. doi: 10.1139/m71-124. [DOI] [PubMed] [Google Scholar]
- GLICK D., FERGUSON R. B., GREENBERG L. J., HALBERG F. Circadian studies on succinic dehydrogenase pantothenate and biotin of rodent adrenal. Am J Physiol. 1961 Apr;200:811–814. doi: 10.1152/ajplegacy.1961.200.4.811. [DOI] [PubMed] [Google Scholar]
- GLICK J. L., COHEN W. D. NOCTURNAL CHANGES IN OXIDATIVE ACTIVITIES OF RAT LIVER MITOCHONDRIA. Science. 1964 Mar 13;143(3611):1184–1185. doi: 10.1126/science.143.3611.1184. [DOI] [PubMed] [Google Scholar]
- Hochberg M. L., Sargent M. L. Regulation of repressible alkaline phosphatase by organic acids and metal ions in Neurospora crassa. Can J Microbiol. 1973 Dec;19(12):1487–1492. doi: 10.1139/m73-242. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NASON A. Neurospora diphosphopyridine nucleotidase. J Biol Chem. 1951 Aug;191(2):473–483. [PubMed] [Google Scholar]
- KUO M. H., BLUMENTHAL H. J. Purification and properties of an acid phosphomonoesterase from Neurospora crassa. Biochim Biophys Acta. 1961 Sep 2;52:13–29. doi: 10.1016/0006-3002(61)90899-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lechner J. F., Fuscaldo K. E., Bazinet G. Genetic and biochemical studies of the hexose monophosphate shunt in Neurospora crassa. II. Characterization of biochemical defects of the morphological mutants colonial 2 and colonial 3. Can J Microbiol. 1971 Jun;17(6):789–794. doi: 10.1139/m71-125. [DOI] [PubMed] [Google Scholar]
- Martens C. L., Sargent M. L. Circadian rhythms of nucleic acid metabolism in Neurospora crassa. J Bacteriol. 1974 Mar;117(3):1210–1215. doi: 10.1128/jb.117.3.1210-1215.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Nyc J. F., Kadner R. J., Crocken B. J. A repressible alkaline phosphatase in Neurospora crassa. J Biol Chem. 1966 Apr 10;241(7):1468–1472. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Pavlidis T., Kauzmann W. Toward a quantitative biochemical model for circadian oscillators. Arch Biochem Biophys. 1969 Jun;132(1):338–348. doi: 10.1016/0003-9861(69)90371-3. [DOI] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R. The effects of light on a circadian rhythm of conidiation in neurospora. Plant Physiol. 1967 Nov;42(11):1504–1510. doi: 10.1104/pp.42.11.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R., Woodward D. O. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 1966 Oct;41(8):1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Kaltenborn S. H. Effects of medium composition and carbon dioxide on circadian conidiation in neurospora. Plant Physiol. 1972 Jul;50(1):171–175. doi: 10.1104/pp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Woodward D. O. Genetic determinants of circadian rhythmicity in Neurospora. J Bacteriol. 1969 Feb;97(2):861–866. doi: 10.1128/jb.97.2.861-866.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren R. E., Romano A. H. Evidence for multiple forms of isocitrate lyase in Neurospora crassa. J Bacteriol. 1967 May;93(5):1638–1643. doi: 10.1128/jb.93.5.1638-1643.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slakey L. L., Craig M. C., Beytia E., Briedis A., Feldbruegge D. H., Dugan R. E., Qureshi A. A., Subbarayan C., Porter J. W. The effects of fasting, refeeding, and time of day on the levels of enzymes effecting the conversion of -hydroxy- -methylglutaryl-coenzyme A to squalene. J Biol Chem. 1972 May 25;247(10):3014–3022. [PubMed] [Google Scholar]
- Stine G. J., Clark A. M. Synchronous production of conidiophores and conidia of Neurospora crassa. Can J Microbiol. 1967 May;13(5):447–453. doi: 10.1139/m67-060. [DOI] [PubMed] [Google Scholar]
- Stine G. J. Enzyme activities during the asexual cycle of Neurospora crassa. 3. Nicotinamide adenosine diphosphate glycohydrolase. Can J Microbiol. 1969 Nov;15(11):1249–1254. doi: 10.1139/m69-227. [DOI] [PubMed] [Google Scholar]
- Stine G. J. Enzyme activities during the asexual cycle of Neurospora crassa. I. Succinic dehydrogenase. Can J Microbiol. 1967 Sep;13(9):1203–1210. doi: 10.1139/m67-165. [DOI] [PubMed] [Google Scholar]
- Stine G. J. Enzyme activities during the asexual cycle of Neurospora crassa. II. NAD- and NADP-dependent glutamic dehydrogenases and nicotinamide adenine dinucleotidase. J Cell Biol. 1968 Apr;37(1):81–88. doi: 10.1083/jcb.37.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURIAN G. [Acetate and its double effect of isocitratase induction and conidial differentiation in Neurospora]. C R Hebd Seances Acad Sci. 1961 Feb 27;252:1374–1376. [PubMed] [Google Scholar]
- Tuveson R. W., West D. J., Barratt R. W. Glutamic acid dehydrogenases in quiescent and germinating conidia of Neurospora crassa. J Gen Microbiol. 1967 Aug;48(2):235–248. doi: 10.1099/00221287-48-2-235. [DOI] [PubMed] [Google Scholar]
- Urey J. C. Enzyme patterns and protein synthesis during synchronous conidiation in Neurospora crassa. Dev Biol. 1971 Sep;26(1):17–27. doi: 10.1016/0012-1606(71)90103-5. [DOI] [PubMed] [Google Scholar]
- Weiss B., Turian G. A study of conidiation in Neurospora crassa. J Gen Microbiol. 1966 Sep;44(3):407–418. doi: 10.1099/00221287-44-3-407. [DOI] [PubMed] [Google Scholar]