Abstract
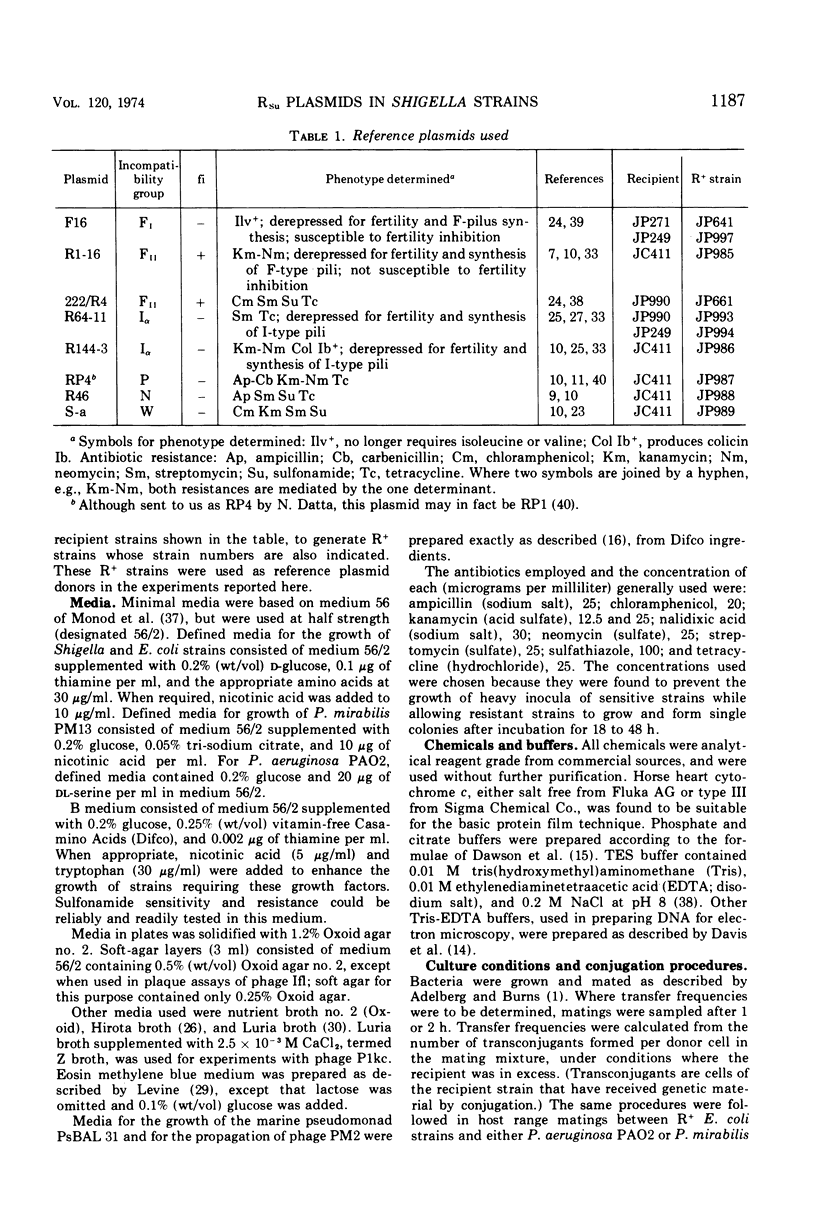
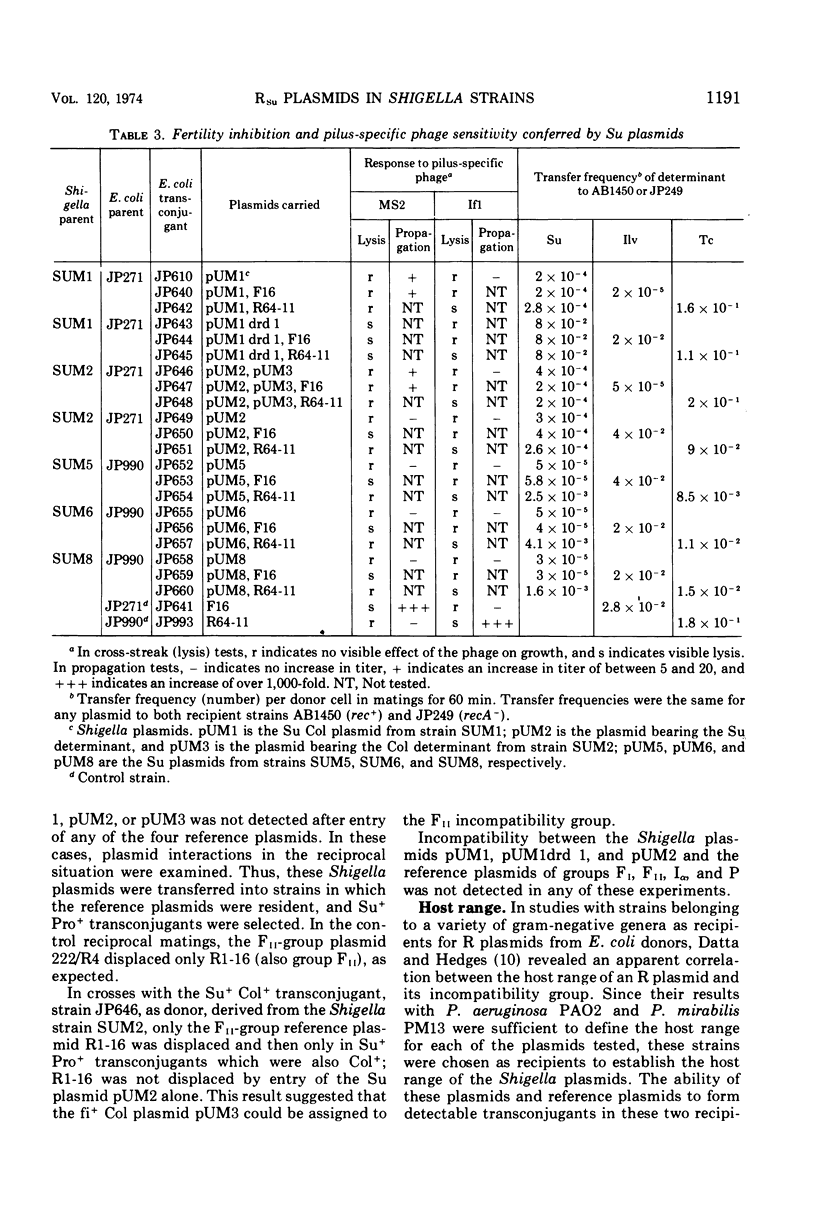
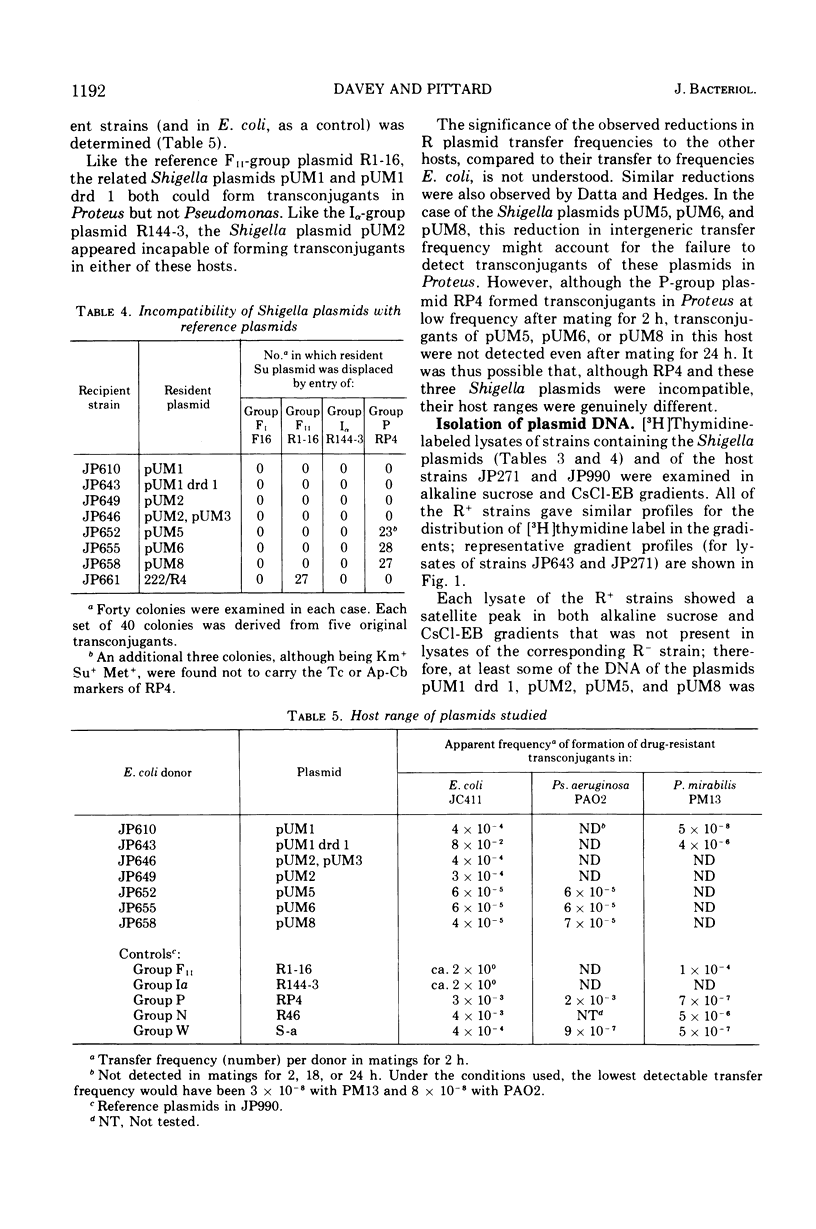
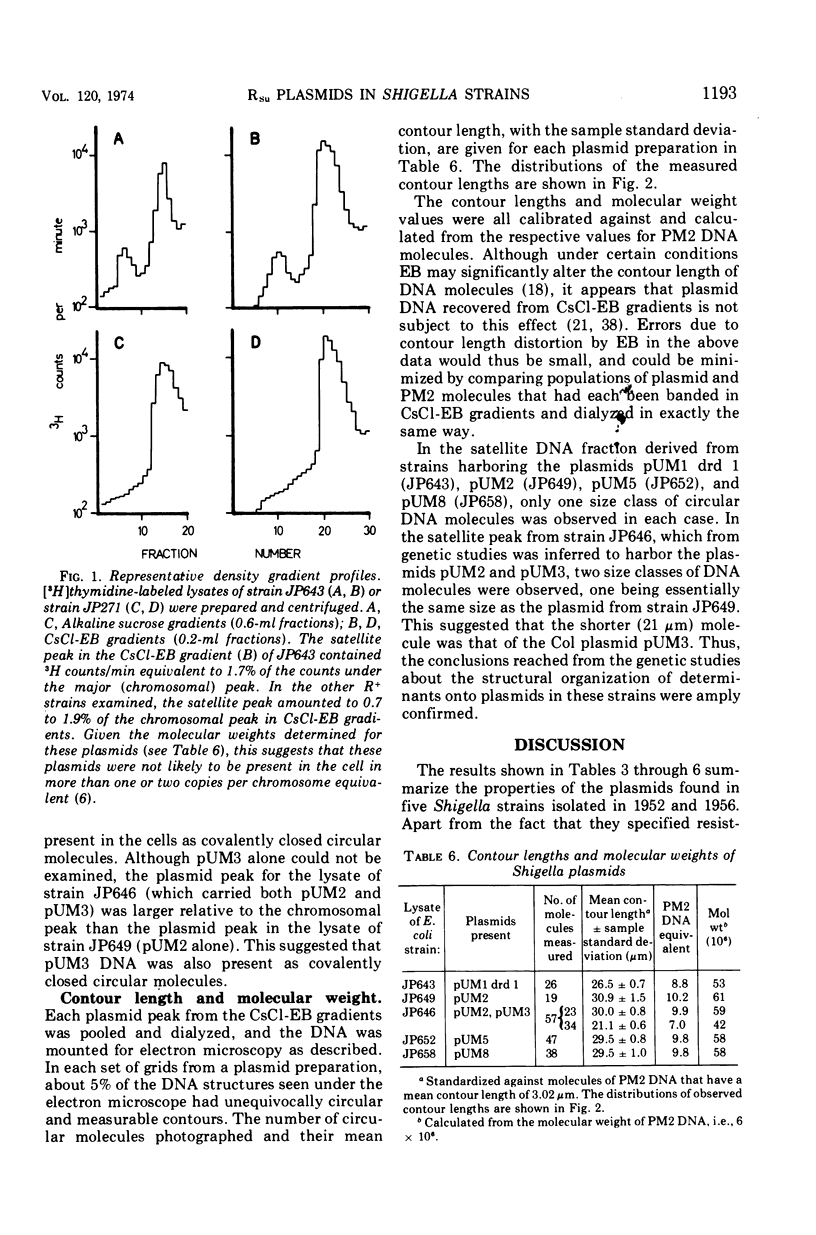
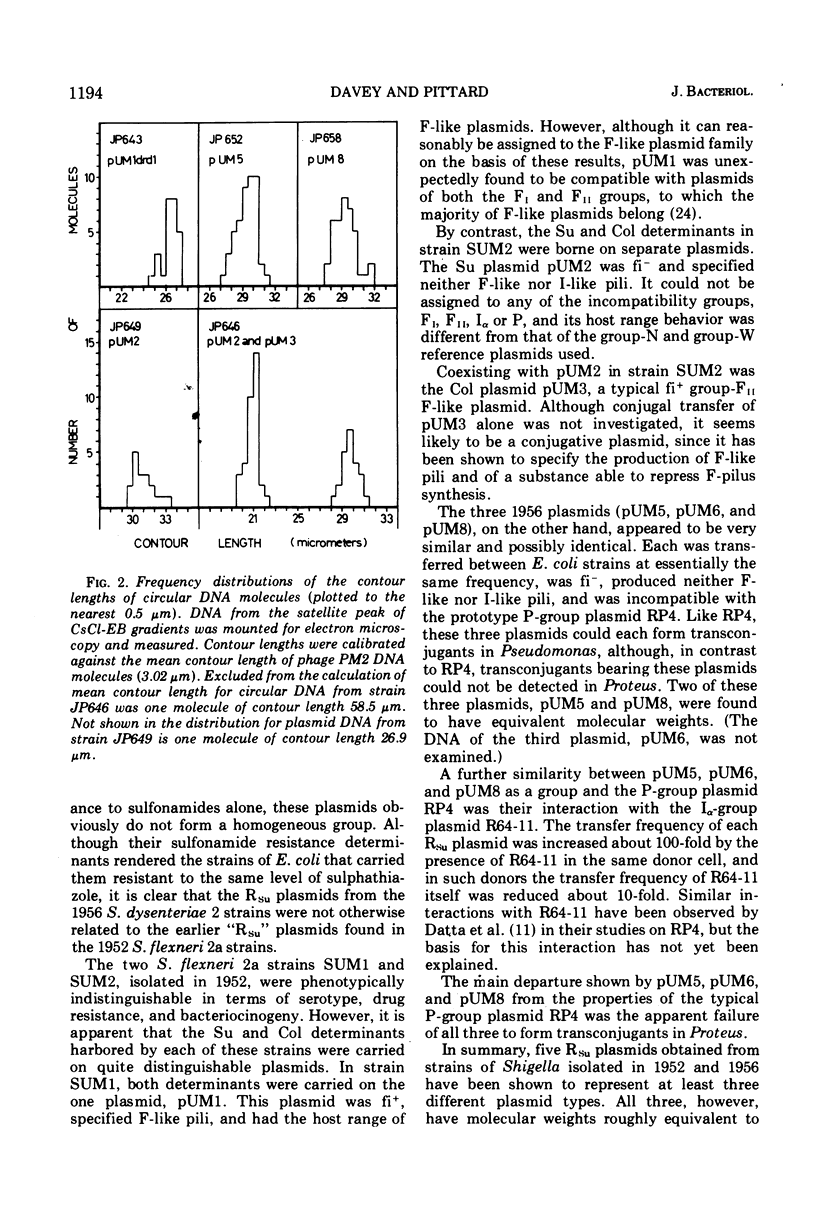
The conjugative plasmids determining sulfonamide resistance in five Shigella strains, each isolated from a different patient, have been characterized. One S. flexneri 2a strain, isolated in 1952, harbored an fi+ plasmid of molecular weight 53 × 106, which specified synthesis of F-like pili and bore determinants for sulfonamide resistance (Su) and bacteriocinogeny (Col). This plasmid was compatible with plasmids of groups FI, FII, Iα, and P. A second S. flexneri 2a strain isolated in 1952 harbored an fi− plasmid of molecular weight 59 × 106, bearing the Su determinant and compatible with all plasmids tested. This strain also harbored an fi+ group-FII plasmid of molecular weight 42 × 106, which bore the Col determinant and specified synthesis of F-like pili. Three S. dysenteriae 2 strains isolated in 1956 carried apparently identical fi− plasmids of molecular weight 58 × 106, which bore the Su determinant, could form transconjugants in Pseudomonas but not in Proteus, and were incompatible with the P-group plasmid RP4.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A map of four genes specifying enzymes involved in catabolism of nucleosides and deoxynucleosides in Escherichia coli. Mol Gen Genet. 1969 Aug 15;104(4):351–359. doi: 10.1007/BF00334234. [DOI] [PubMed] [Google Scholar]
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M., Meynell E., Lawn A. M. Mutant Hfr strains defective in transfer: restoration by F-like and I-like de-repressed R factors. Genet Res. 1970 Aug;16(1):101–112. doi: 10.1017/s0016672300002317. [DOI] [PubMed] [Google Scholar]
- Datta N. Drug resistance and R factors in the bowel bacteria of London patients before and after admission to hospital. Br Med J. 1969 May 17;2(5654):407–411. doi: 10.1136/bmj.2.5654.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R. B., Pittard A. J. Transferable multiple antibiotic resistance amongst shigella strains isolated in melbourne between 1952 and 1968. Med J Aust. 1971 Jun 26;1(26):1367–1370. doi: 10.5694/j.1326-5377.1971.tb50333.x. [DOI] [PubMed] [Google Scholar]
- Davies J. R., Farrant W. N., Tomlinson A. J. Further studies on the antibiotic resistance of Shigella sonnei. I. Transferable antibiotic resistance. J Hyg (Lond) 1968 Sep;66(3):471–477. doi: 10.1017/s0022172400041334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Electron microscopic study of the ethidium bromide-DNA complex. J Mol Biol. 1971 Sep 14;60(2):401–403. doi: 10.1016/0022-2836(71)90303-2. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Humphreys G. O., Anderson E. S. Molecular studies of R factor compatibility groups. J Bacteriol. 1973 Jul;115(1):387–398. doi: 10.1128/jb.115.1.387-398.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn F. E., Ciak J. The problems of drug-resistant pathogenic bacteria. Elimination of bacterial episomes by DNA-complexing compounds. Ann N Y Acad Sci. 1971 Jun 11;182:295–304. doi: 10.1111/j.1749-6632.1971.tb30665.x. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. Plasmids determining I pili constitute a compatibility complex. J Gen Microbiol. 1973 Jul;77(1):19–25. doi: 10.1099/00221287-77-1-19. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- Levine M. The Effect of Concentration of Dyes on Differentiation of Enteric Bacteria on Eosin-Methylene-Blue Agar. J Bacteriol. 1943 May;45(5):471–475. doi: 10.1128/jb.45.5.471-475.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell G. G. Dodecylamine in the isolation of bacterial DNA. Biochim Biophys Acta. 1971 Jun 17;240(1):37–38. doi: 10.1016/0005-2787(71)90509-0. [DOI] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Filamentous phages specific for the I sex factor. Nature. 1968 Mar 23;217(5134):1184–1186. doi: 10.1038/2171184a0. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. III. AN ANALYSIS OF THE RECOMBINATION EVENTS OCCURRING IN THE F' MALE AND IN THE ZYGOTES. Genetics. 1964 Jun;49:995–1007. doi: 10.1093/genetics/49.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. R., Grinsted J. Properties of RP4, an R factor which originated in Pseudomonas aeruginosa S8. J Bacteriol. 1972 Nov;112(2):690–696. doi: 10.1128/jb.112.2.690-696.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


