Abstract
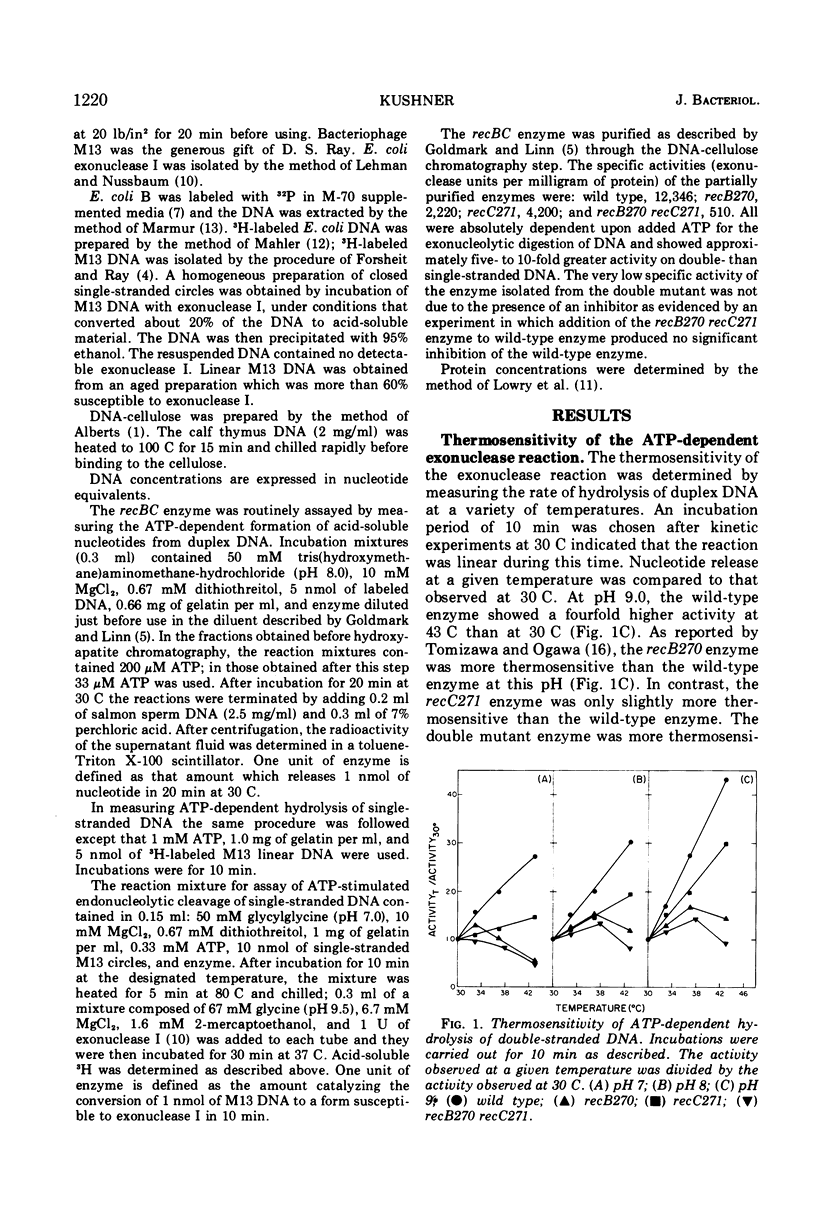
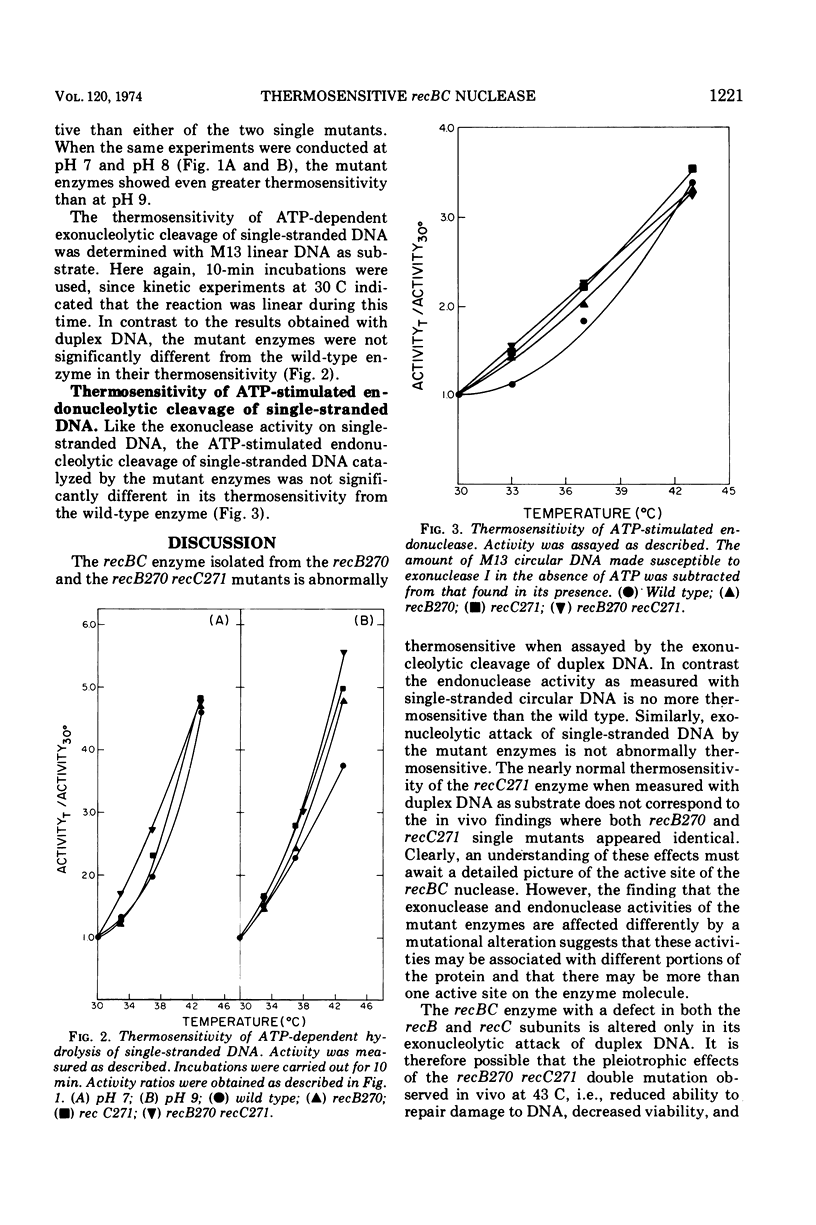
The recBC nuclease (also called exonuclease V) has been partially purified from Escherichia coli K-12 strains carrying the thermosensitive recB270, recC271, and recB270 recC271 mutations. Of the multiple activities associated with the enzyme, only the adenosine 5′-triphosphate-dependent exonucleolytic hydrolysis of duplex deoxyribonucleic acid (DNA) is abnormally thermolabile. The exo- and endonucleolytic degradation of single-stranded DNA is no more thermosensitive than that catalyzed by the wild-type enzyme. These results suggest that the defects in genetic recombination, DNA repair, and the maintenance of cell viability observed in recBC mutants in vivo result primarily from the specific loss of adenosine 5′-triphosphate-dependent exonuclease active on duplex DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsheit A. B., Ray D. S. Conformations of the single-stranded DNA of bacteriophage M13. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1534–1541. doi: 10.1073/pnas.67.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Haefner K. Spontaneous lethal sectoring, a further feature of Escherichia coli strains deficient in the function of rec and uvr genes. J Bacteriol. 1968 Sep;96(3):652–659. doi: 10.1128/jb.96.3.652-659.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D. H., Hayes F., Guérin M. F. Association of rapidly labelled bacterial RNA with ribosomal RNA in solutions of high ionic strength. II. J Mol Biol. 1966 Jul;18(3):499–515. doi: 10.1016/s0022-2836(66)80039-6. [DOI] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. doi: 10.1016/0022-2836(72)90215-x. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Rola F. H., Pasetto-Nobrega M., Oishi M. Adenosine triphosphatase associated with adenosine triphosphate-dependent deoxyribonuclease (recB-recC enzyme-E. coli-ATP to phosphodiester hydrolysis ratio-DNA-dependent ATPase activity). Proc Natl Acad Sci U S A. 1972 Jan;69(1):15–19. doi: 10.1073/pnas.69.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]


