Abstract
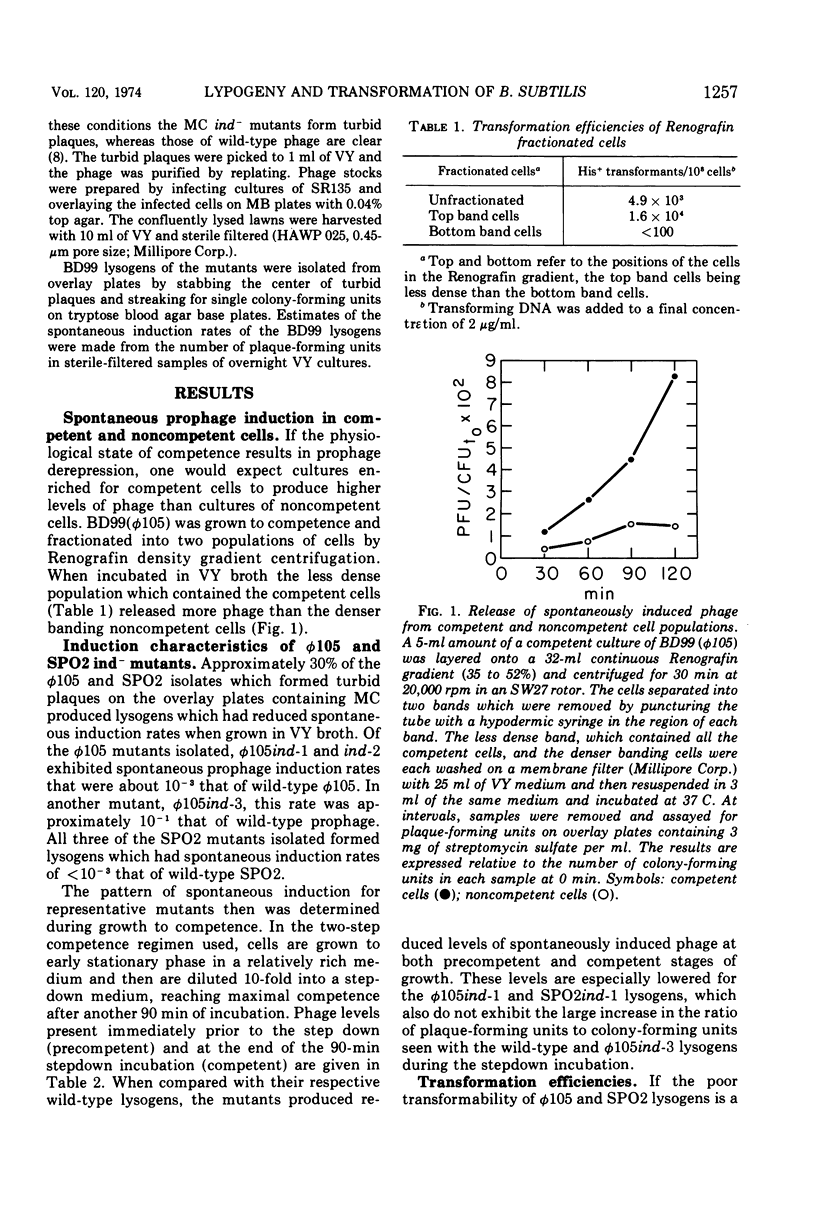
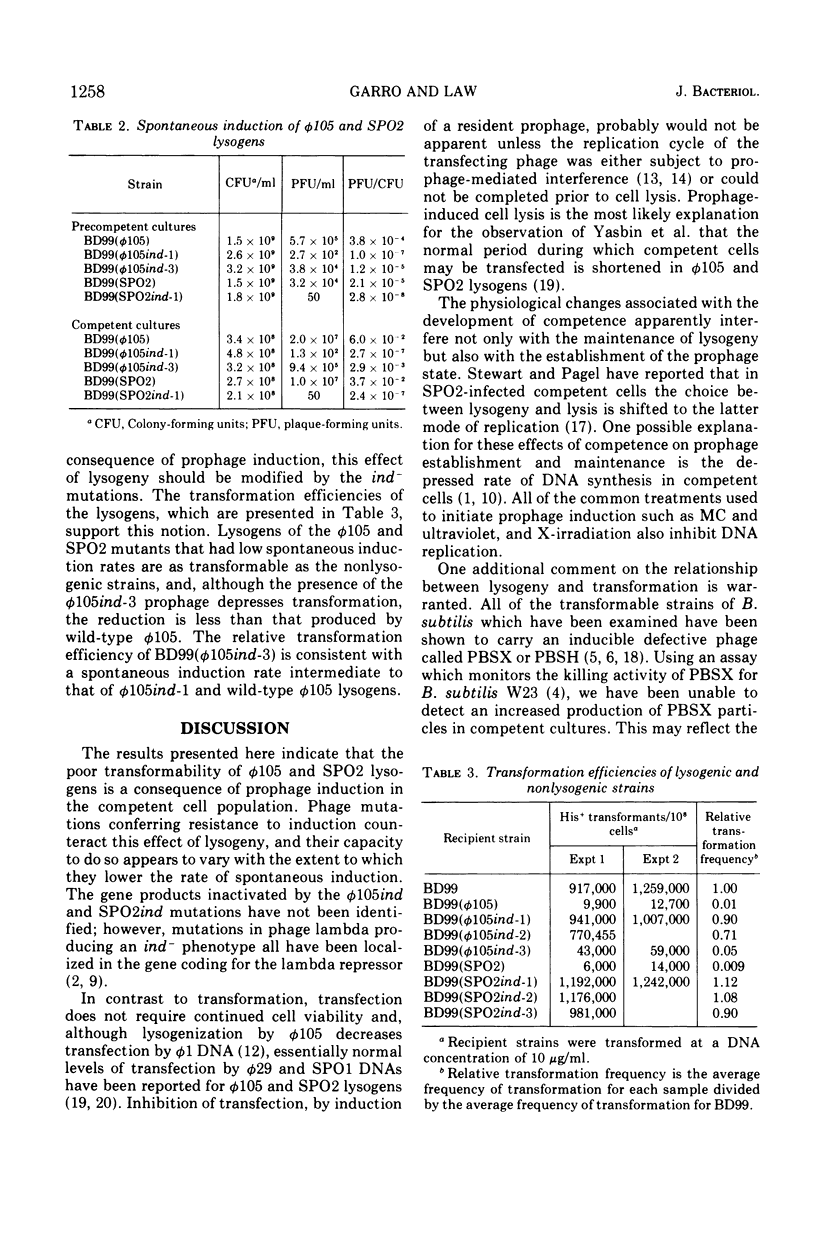
The low transformation efficiency of Bacillus subtilis 168 lysogenic for phages ø105 or SPO2 is shown to result from the induction of lytic phage replication in competent cells. Lysogenic competent cells have a higher rate of spontaneous prophage induction than noncompetent cells. Mutants of ø105 and SPO2 which form lysogens resistant to spontaneous induction were isolated, and these lysogens exhibited higher transformation levels than those formed by wild-type phage. These results suggest that the physiological state of competence in B. subtilis promotes prophage derepression leading to cell death and the loss of potential transformants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dooley D. C., Hadden C. T., Nester E. W. Macromolecular synthesis in Bacillus subtilis during development of the competent state. J Bacteriol. 1971 Nov;108(2):668–679. doi: 10.1128/jb.108.2.668-679.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J. Isolation and properties of Bacillus subtilis strains lysogenized by a clear plaque mutant of bacteriophage phi 105. J Virol. 1973 Jul;12(1):13–17. doi: 10.1128/jvi.12.1.13-17.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Leffert H., Marmur J. Genetic mapping of a defective bacteriophage on the chromosome of Bacillus subtilis 168. J Virol. 1970 Sep;6(3):340–343. doi: 10.1128/jvi.6.3.340-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Marmur J. Defective bacteriophages. J Cell Physiol. 1970 Dec;76(3):253–263. doi: 10.1002/jcp.1040760305. [DOI] [PubMed] [Google Scholar]
- Haas M., Yoshikawa H. Defective bacteriophage PBSH in Bacillus subtilis. I. Induction, purification, and physical properties of the bacteriophage and its deoxyribonucleic acid. J Virol. 1969 Feb;3(2):233–247. doi: 10.1128/jvi.3.2.233-247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T., Inokuchi H. Temperature-sensitive regulation system of prophage lambda induction. J Mol Biol. 1967 Jan 28;23(2):217–224. doi: 10.1016/s0022-2836(67)80029-9. [DOI] [PubMed] [Google Scholar]
- JACOB F., CAMPBELL A. Sur le système de répression assurant l'immunité chez les bactéries lysogenes. C R Hebd Seances Acad Sci. 1959 Jun 1;248(22):3219–3221. [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J Bacteriol. 1967 Jul;94(1):131–140. doi: 10.1128/jb.94.1.131-140.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. M., Rutberg L. Linked transformation of bacterial and prophage markers in Bacillus subtilis 168 lysogenic for bacteriophage phi 105. J Bacteriol. 1969 Jun;98(3):874–877. doi: 10.1128/jb.98.3.874-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Abortive infection of lysogenic Bacillus subtilis 168(SPO2) by bacteriophage phi 1. J Virol. 1974 Apr;13(4):870–880. doi: 10.1128/jvi.13.4.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Prophage-mediated interference affecting the development of Bacillus subtilis bacteriophage phi e. J Virol. 1973 Mar;11(3):372–377. doi: 10.1128/jvi.11.3.372-377.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner R., Remeza V. Chromatographically fractionated complementary strands of Bacillus subtilis deoxyribonucleic acid: biological properties. J Bacteriol. 1973 Feb;113(2):739–753. doi: 10.1128/jb.113.2.739-753.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L. Mapping of a temperate bacteriophage active on Bacillus subtilis. J Virol. 1969 Jan;3(1):38–44. doi: 10.1128/jvi.3.1.38-44.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Pagel M. F. Relationship between transformation in Bacillus subtilis and infection by bacteriophage SP02. J Bacteriol. 1973 Nov;116(2):1082–1083. doi: 10.1128/jb.116.2.1082-1083.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Young F. E. The influence of temperate bacteriophage phi105 on transformation and transfection in Bacillus subtilis. Biochem Biophys Res Commun. 1972 Apr 28;47(2):365–371. doi: 10.1016/0006-291x(72)90722-x. [DOI] [PubMed] [Google Scholar]



