Abstract
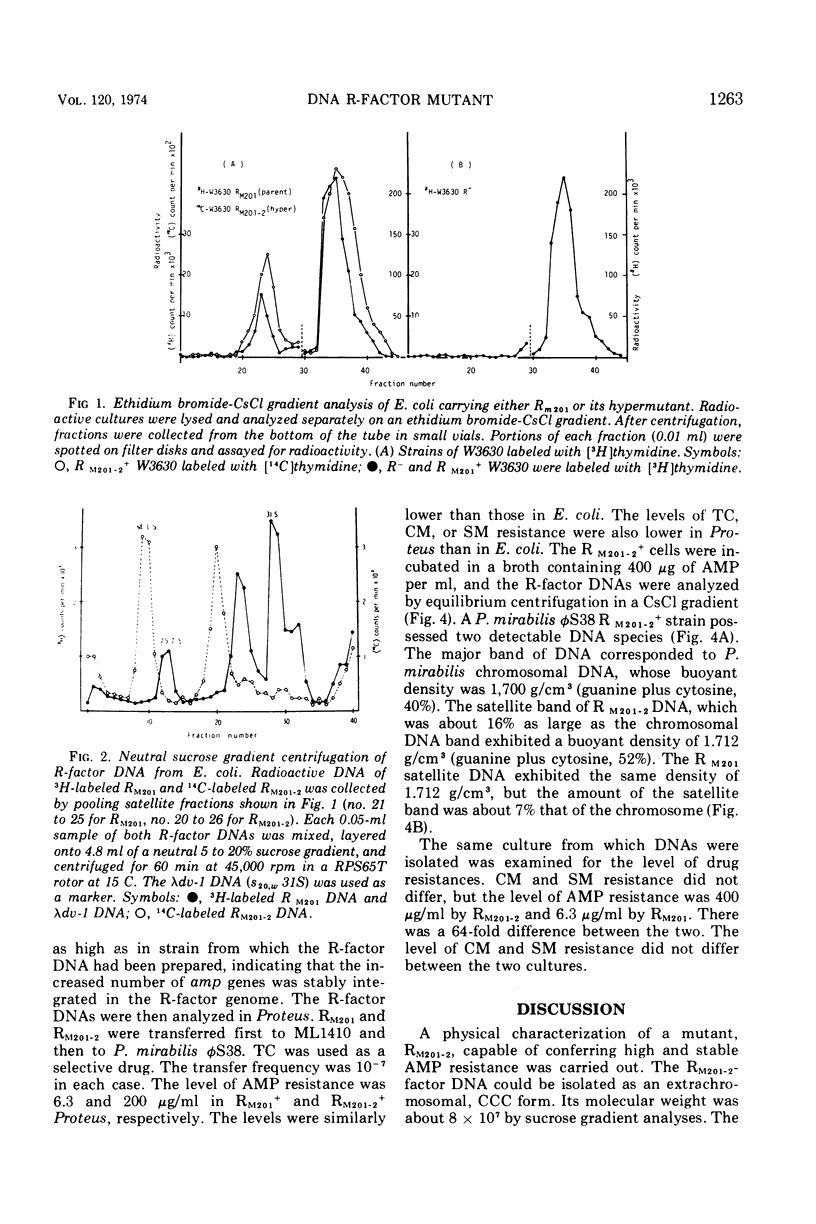
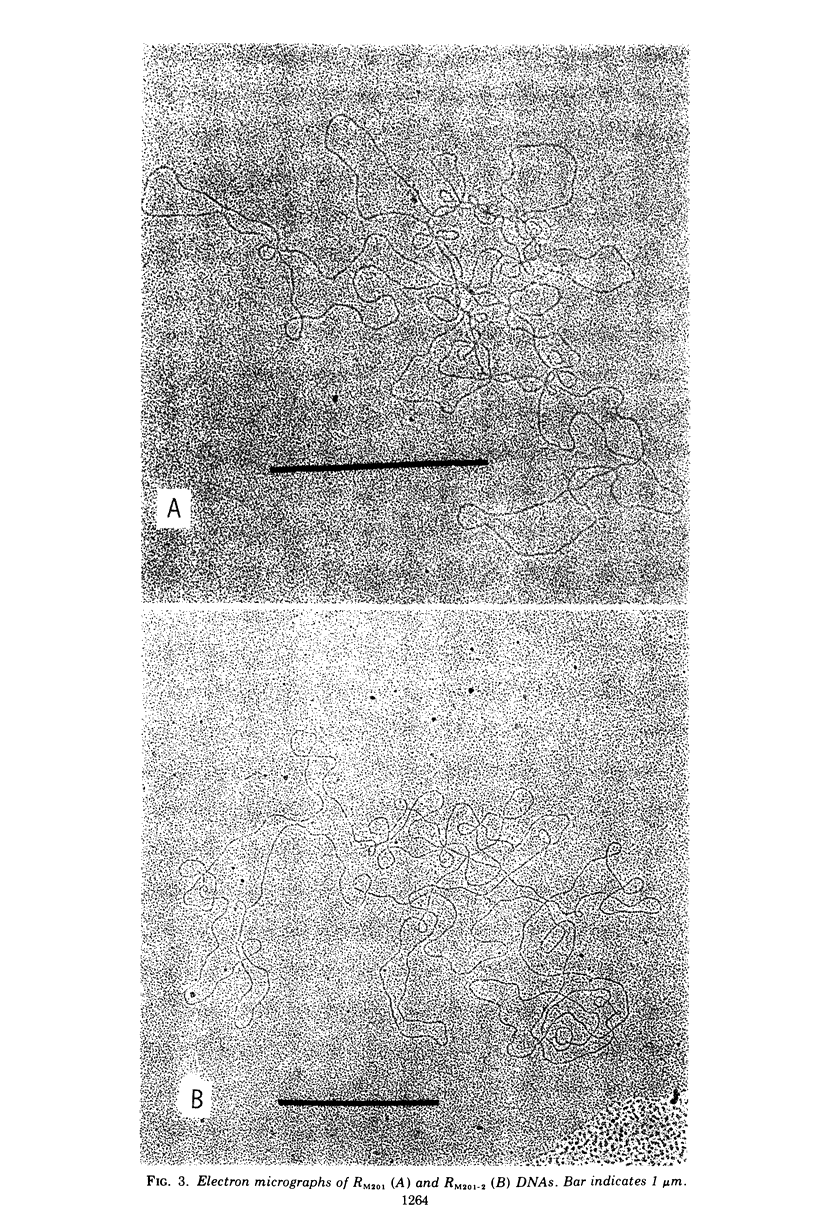
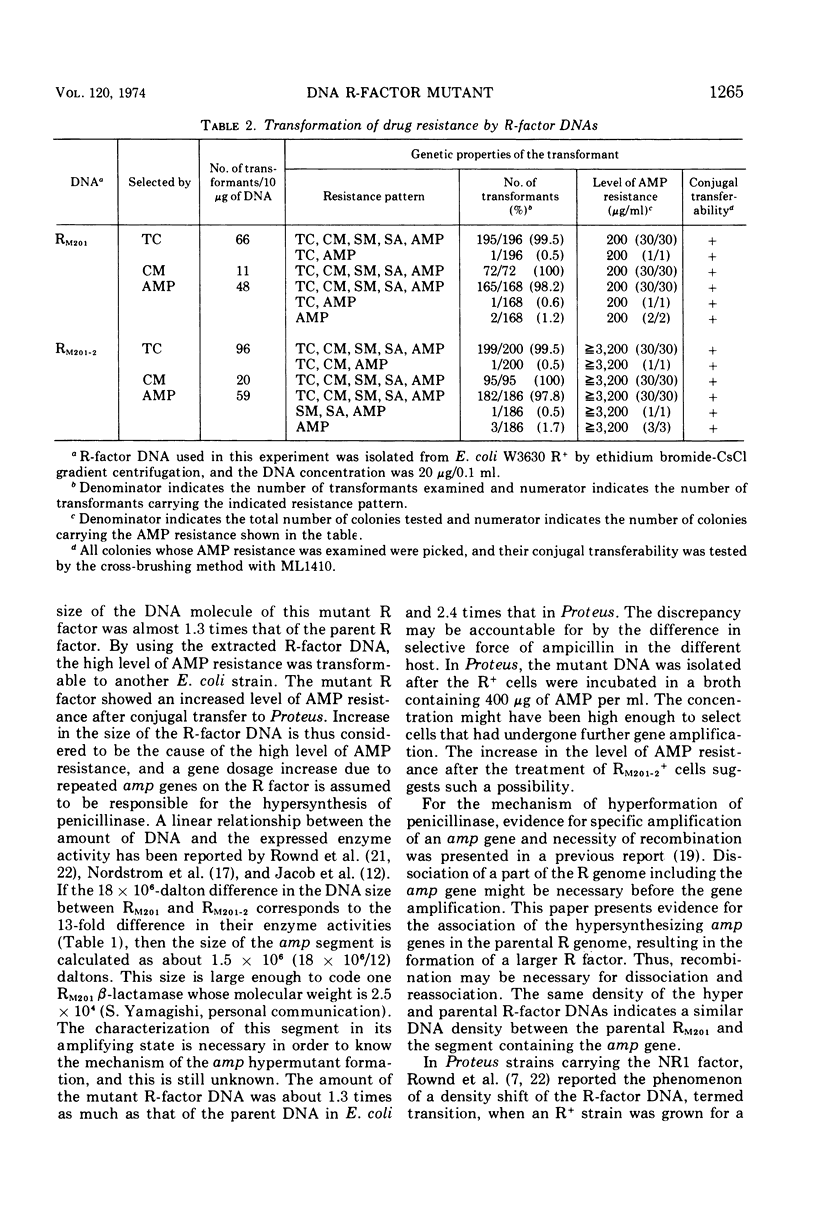
The physical characteristics of a mutant, RM201-2, capable of conferring high and stable ampicillion resistance was analyzed. The RM201-2 and its parent R-factor deoxyribonucleic acid (DNA) could be isolated as an extrachromosomal and covalently closed circular form. Their buoyant densities were both 1.712 g/cm3, and their molecular weights were about 82 × 106 and 64 × 106, respectively, when measured by CsCl and sucrose density gradient analyses. The contour lengths by electron microscopy were 35.9 ± 0.6 and 31.0 ± 0.6 μm, respectively. By using the extracted R-factor DNA, the mutant and parent characters were transformable to another Escherichia coli strain. The mutant R factor showed an increased amount of DNA even after conjugal transfer to Proteus. An increase in the size of R-factor DNA was thus considered to be the cause of the high level of ampicillin resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruenn J., Hollingsworth H. A mutant of Escherichia coli with a new, highly efficient promoter for the lactose operon. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3693–3697. doi: 10.1073/pnas.70.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature. 1967 Nov 18;216(5116):652–657. doi: 10.1038/216652a0. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Isolation and characterization of supercoiled circular deoxyribonucleic acid from beta-hemolytic strains of Escherichia coli. J Bacteriol. 1971 May;106(2):311–317. doi: 10.1128/jb.106.2.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Tanaka T., Mitsuhashi S. Genetic structure of an R factor conferring ampicillin resistance. Jpn J Microbiol. 1973 Sep;17(5):331–337. doi: 10.1111/j.1348-0421.1973.tb00783.x. [DOI] [PubMed] [Google Scholar]
- KONDO E., HARADA K., MITSUHASHI S. Drug-resistance of enteric bacteria. 12. Transduction of the transmissible drug resistance factor by bacteriophage Plkc. Jpn J Exp Med. 1962 Feb;32:139–147. [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odakura Y., Tanaka T., Hashimoto H., Mitsuhashi S. Mutation of R factors capable of specifying hypersynthesis of penicillinase. Antimicrob Agents Chemother. 1973 Mar;3(3):315–324. doi: 10.1128/aac.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odakura Y., Tanaka T., Mitsuhashi S. Drug-resistance and distribution of R factors among Proteus strains. Jpn J Microbiol. 1971 Jul;15(4):367–372. doi: 10.1111/j.1348-0421.1971.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Rownd R., Kasamatsu H., Mickel S. The molecular nature and replication of drug resistance factors of the Enterobacteriaceae. Ann N Y Acad Sci. 1971 Jun 11;182:188–206. doi: 10.1111/j.1749-6632.1971.tb30656.x. [DOI] [PubMed] [Google Scholar]
- Rownd R., Perlman D., Hashimoto H., Mickel S., Applebaum E., Taylor D. Dissociation and reassociation of the transfer factor and resistance determinants of R factors as a mechanism of gene amplification in bacteria. Johns Hopkins Med J Suppl. 1973;2:115–128. [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sawai T., Yamagishi S., Mitsuhashi S. Penicillinases of Klebsiella pneumoniae and their phylogenetic relationship to penicillinases mediated by R factors. J Bacteriol. 1973 Sep;115(3):1045–1054. doi: 10.1128/jb.115.3.1045-1054.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]