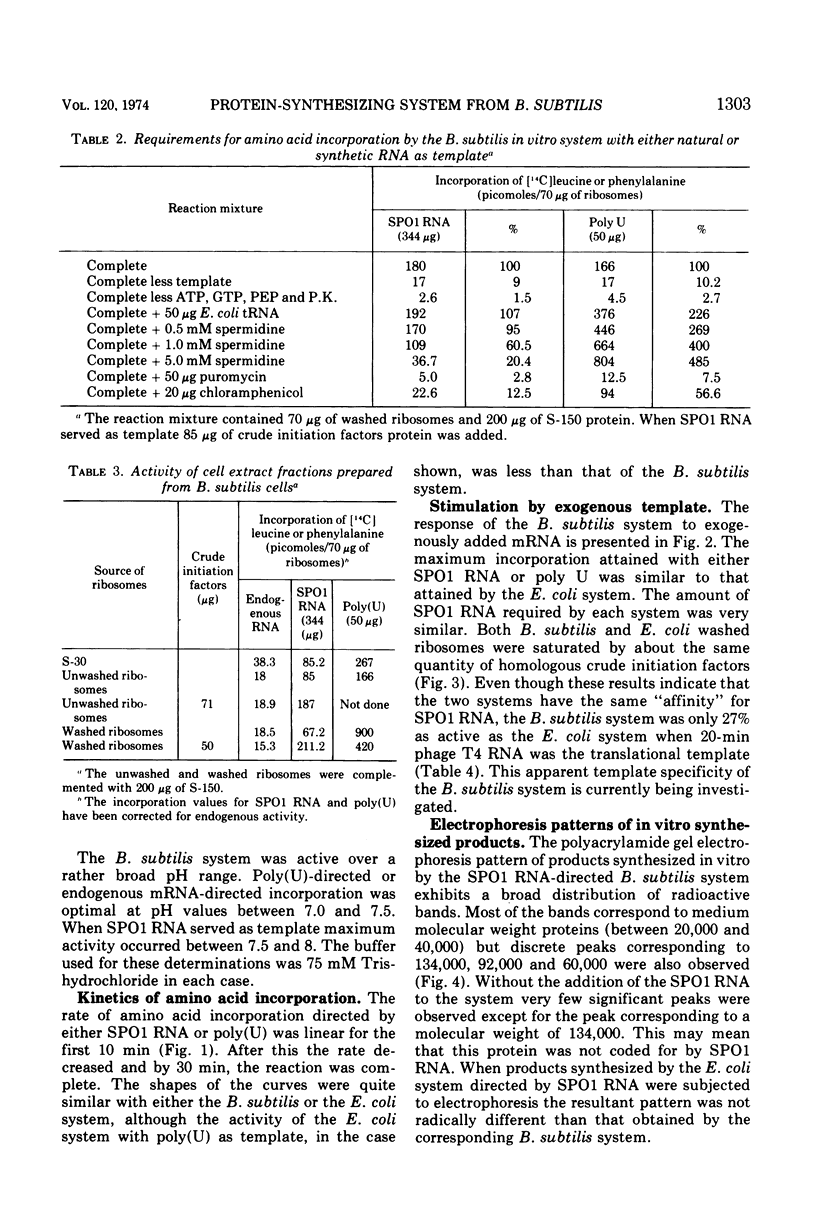
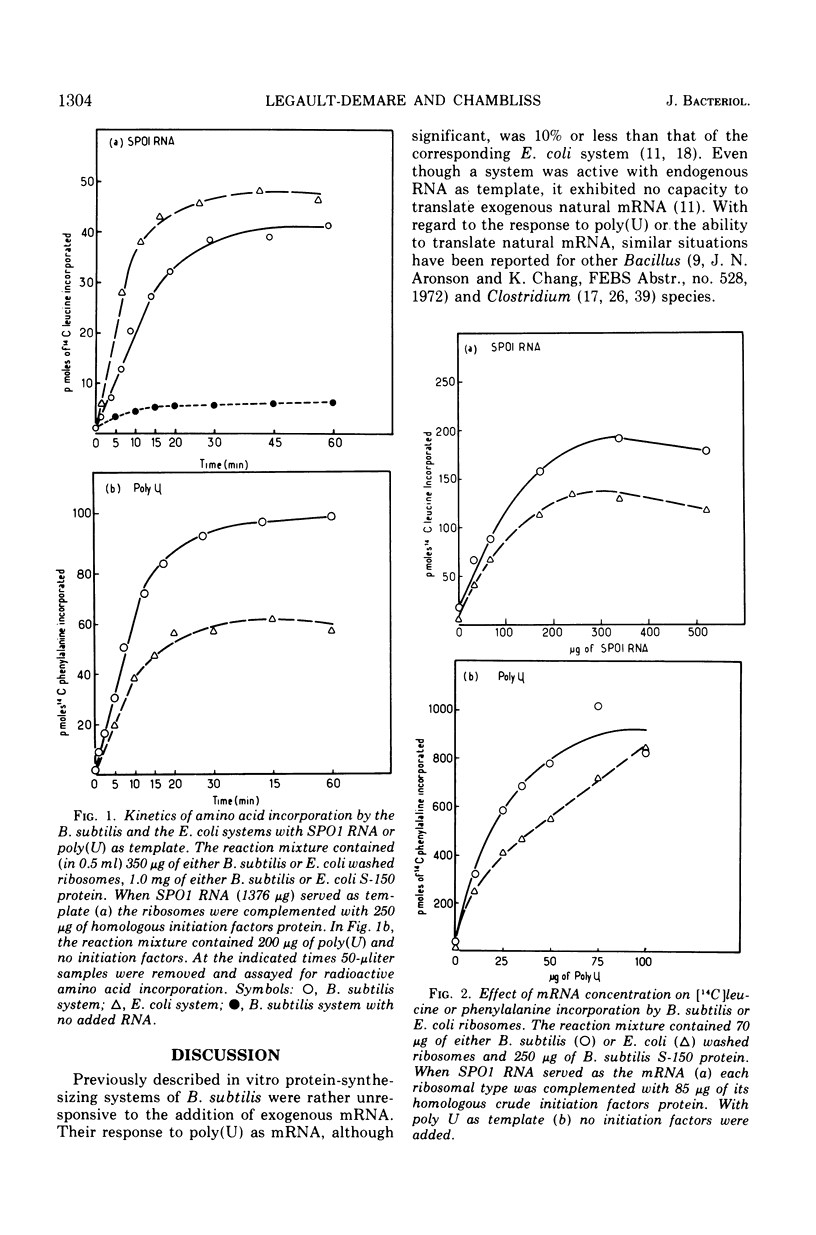
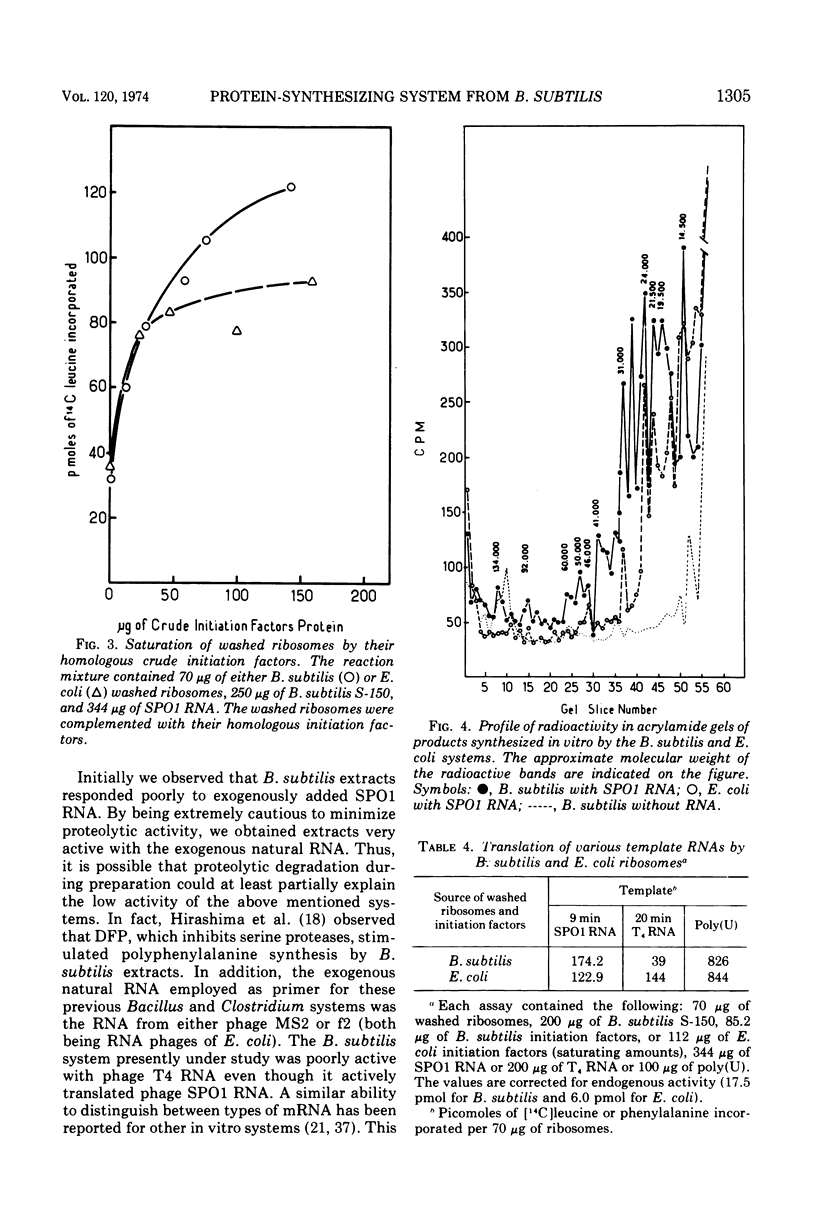
Abstract
A very active in vitro protein-synthesizing system has been developed from Bacillus subtilis. High activity in the extracts is dependent upon precautions taken to reduce proteolytic activity. Endogenous, exogenous natural and synthetic messenger ribonucleic acids (RNAs) are translated by the system. The activity of the B. subtilis system has been compared to that of the Escherichia coli system. With either SPO1 RNA or polyuridylic acid, the activities of the two systems were very similar. Electrophoresis of the products synthesized in vitro by the two systems primed with SPO1 RNA yields similar radioactive profiles. The major bands of radioactivity correspond to proteins of molecular weight between 15,000 and 40,000.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop H. L., Migita L. K., Doi R. H. Peptide synthesis by extracts from Bacillus subtilis spores. J Bacteriol. 1969 Sep;99(3):771–778. doi: 10.1128/jb.99.3.771-778.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Byfield J. E., Scherbaum O. H. A rapid radioassay technique for cellular suspensions. Anal Biochem. 1966 Dec;17(3):434–443. doi: 10.1016/0003-2697(66)90179-5. [DOI] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changchien L. M., Aronson J. N. Spermidine requirement for Bacillus thuringiensis ribosomes in cell-free phenylalanine incorporation. J Bacteriol. 1970 Sep;103(3):734–740. doi: 10.1128/jb.103.3.734-740.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Chambon P., Konberg A. Biochemical studies of bacterial sporulation and germination. XI. Protein-synthesizing systems from vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5117–5125. [PubMed] [Google Scholar]
- Fortnagel P., Bergmann R. Alteration of the ribosomal fraction of Bacillus subtilis during sporulation. Biochim Biophys Acta. 1973 Feb 23;299(1):136–141. doi: 10.1016/0005-2787(73)90404-8. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Guha S., Szulmajster J. Effect of fusidic acid on sporulation of Bacillus subtilis. FEBS Lett. 1974 Jan 15;38(3):315–319. doi: 10.1016/0014-5793(74)80081-5. [DOI] [PubMed] [Google Scholar]
- Himes R. H., Stallcup M. R., Rabinowitz J. C. Translation of synthetic and endogenous messenger ribonucleic acid in vitro by ribosomes and polyribosomes from Clostridium pasteurianum. J Bacteriol. 1972 Dec;112(3):1057–1069. doi: 10.1128/jb.112.3.1057-1069.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUCAN Z., LIPMANN F. DIFFERENCES IN CHLORAMPHENICOL SENSITIVITY OF CELL-FREE AMINO ACID POLYMERIZATION SYSTEMS. J Biol Chem. 1964 Feb;239:516–520. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legault-Demare L., Jeantet C., Gros F. Metabolic fate of initiation factors after inhibition of protein synthesis in Escherichia coli. Mol Gen Genet. 1973 Sep 27;125(4):301–318. doi: 10.1007/BF00276586. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Ames B. N. THE EFFECT OF POLYAMINES AND OF POLY U SIZE ON PHENYLALANINE INCORPORATION. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2171–2178. doi: 10.1073/pnas.48.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepokroeff C., Aronson A. I. Cell-free synthesis of ferredoxin in clostridial extracts. Biochemistry. 1970 May 12;9(10):2074–2081. doi: 10.1021/bi00812a008. [DOI] [PubMed] [Google Scholar]
- Orrego C., Kerjan P., Manca de Nadra M. C., Szulmajster J. Ribonucleic acid polymerase in a thermosensitive sporulation mutant (ts-4) of Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):636–647. doi: 10.1128/jb.116.2.636-647.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., Kaji A. Evidence for specific inhibition of translocation of aminoacyl-transfer ribonucleic acid by the pretreatment of ribosomes of Bacillus subtilis with p-chloromercuribenzoate. J Bacteriol. 1972 Oct;112(1):188–194. doi: 10.1128/jb.112.1.188-194.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Brenowitz J. Translation of MS2 RNA by ribosomes from different bacterial species. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1154–1160. doi: 10.1016/0006-291x(70)90360-8. [DOI] [PubMed] [Google Scholar]
- Takeda M., Lipmann F. Comparison of amino Acid polymerization in B. Subtilis and e. Coli cell-free systems; hybridization of their ribosomes. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1875–1882. doi: 10.1073/pnas.56.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakatellis A. C., Schwartz G. The biosynthesis of ferredoxin in a cell-free system. Proc Natl Acad Sci U S A. 1969 Jun;63(2):436–441. doi: 10.1073/pnas.63.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE A. D., HAHN F. E. MODE OF ACTION OF CHLORAMPHENICOL. IX. EFFECTS OF CHLORAMPHENICOL UPON A RIBOSOMAL AMINO ACID POLYMERIZATION SYSTEM AND ITS BINDING TO BACTERIAL RIBOSOME. Biochim Biophys Acta. 1965 Jan 11;95:146–155. doi: 10.1016/0005-2787(65)90219-4. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


