Abstract
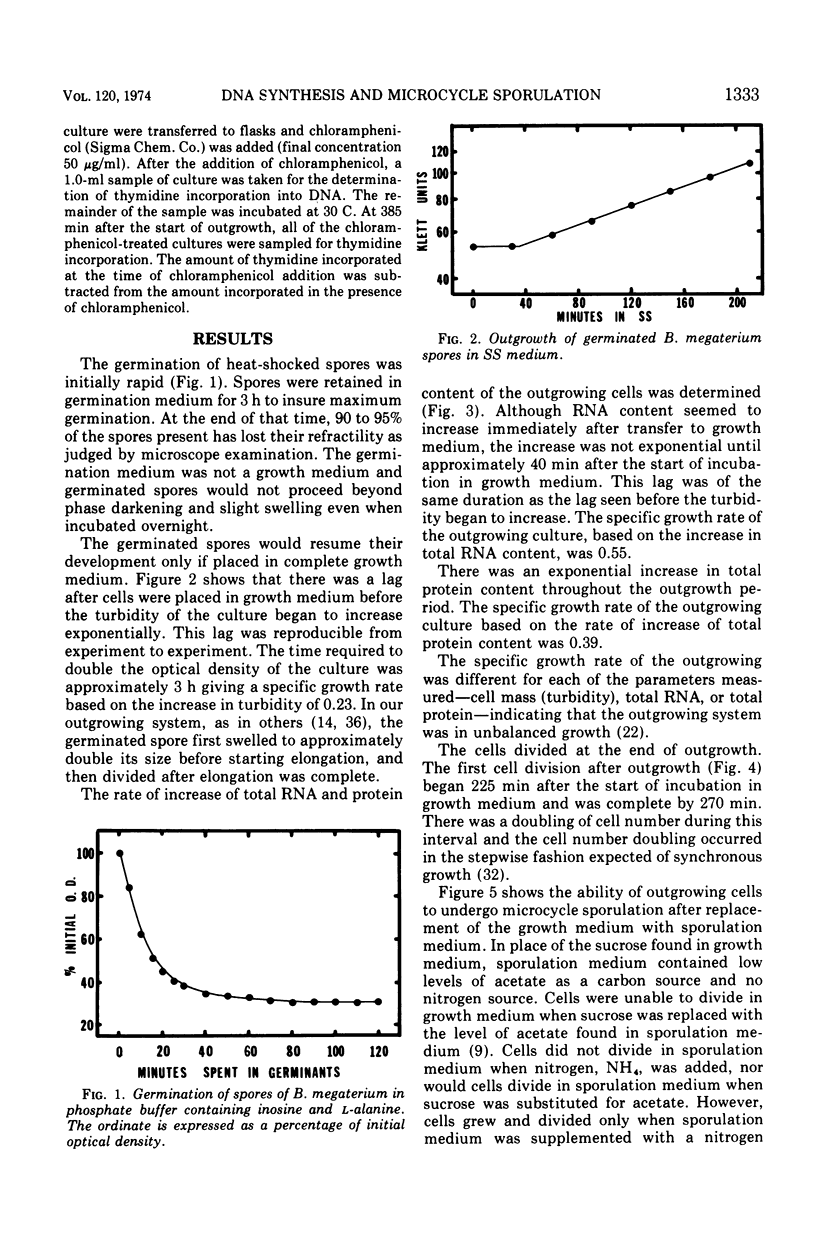
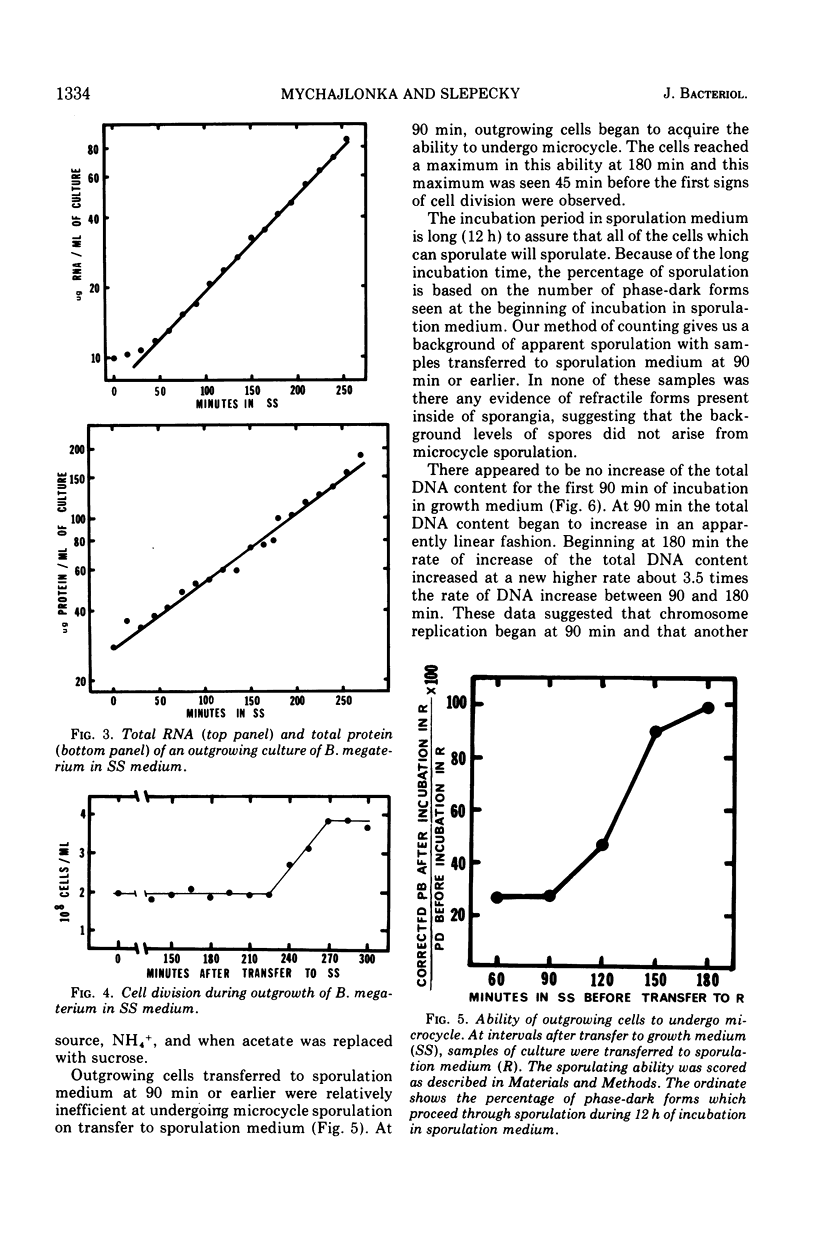
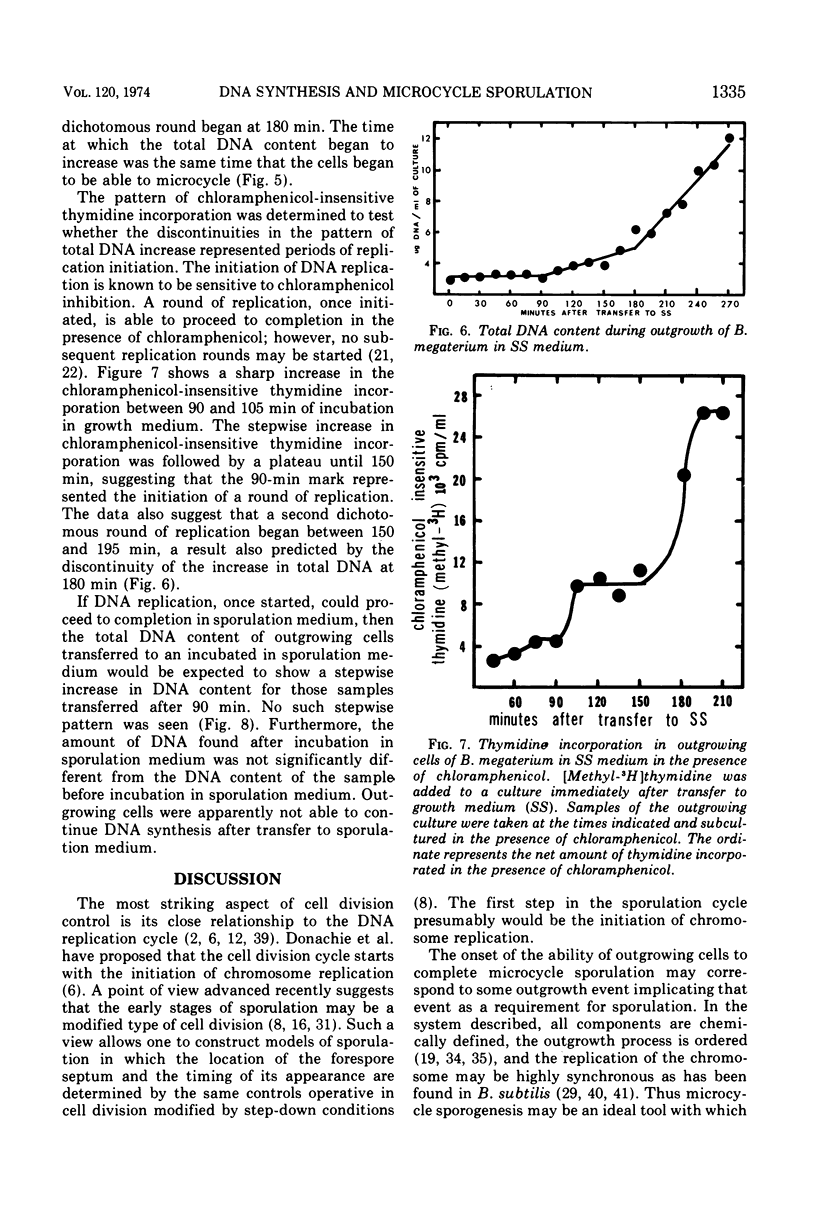
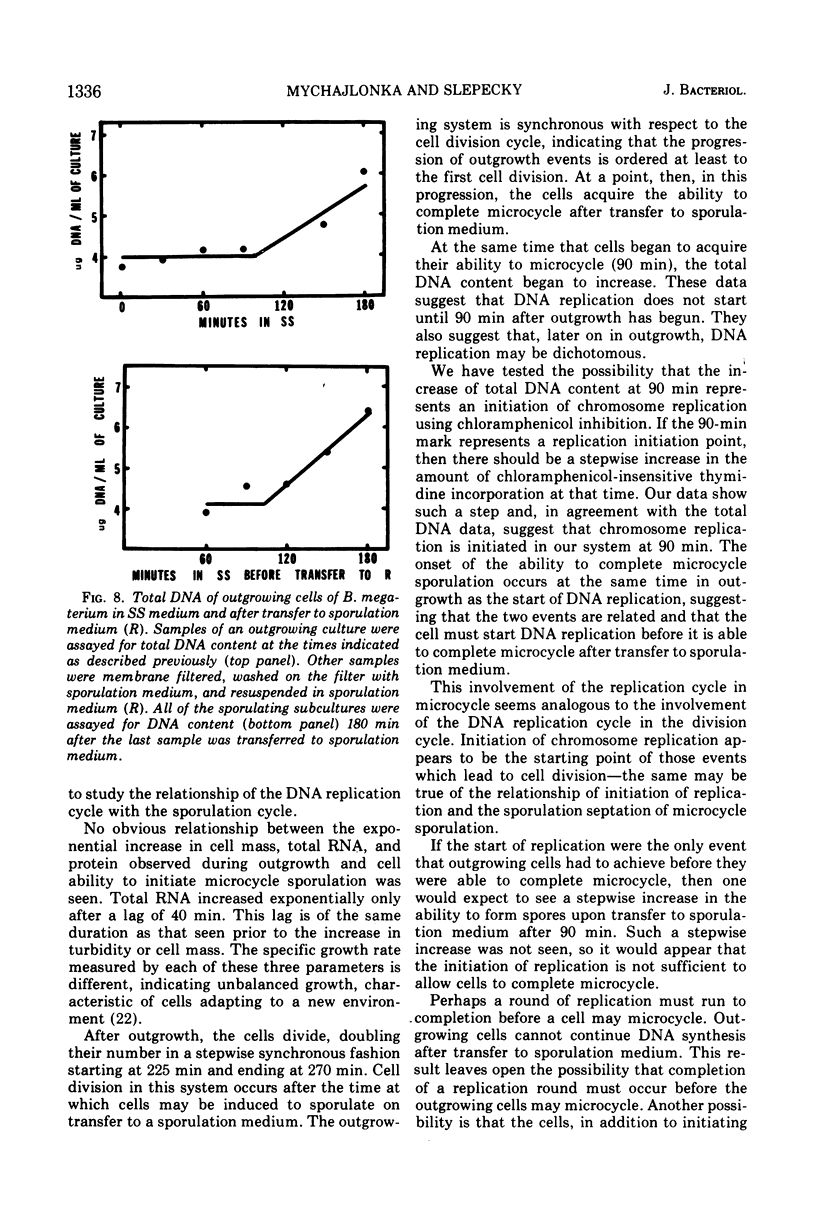
Bacillus megaterium cells have been examined during outgrowth for their macromolecular content, ability to undergo microcycle sporulation, the time of their growth division, the time of deoxyribonucleic acid (DNA) replication initiation, and their ability to synthesize DNA after transfer to sporulation medium. The increase in total DNA content of the cells increased discontinuously beginning at 90 min. Thymidine incorporation became insensitive to chloramphenicol between 90 and 105 min of outgrowth. At 90 min the cells acquired the ability to undergo microcycle sporulation and the degree of sporulation depended on the time spent in outgrowth, with maximal sporulation occurring at 180 min. During outgrowth, cells underwent one synchronous growth division beginning at 225 min and ending at 270 min. Outgrowing cells were not able to continue DNA synthesis after transfer to sporulation medium. The data suggest that DNA replication starts before cells are able to undergo microcycle sporulation; however, the initiation of replication may not be the only requirement for microcycle sporulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. The regulation of DNA replication and cell division in E. coli B-r. Cold Spring Harb Symp Quant Biol. 1968;33:823–838. doi: 10.1101/sqb.1968.033.01.094. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Kay D., Mandelstam J. Determining effect of growth medium on the shape and position of daughter chromosomes and on sporulation in Bacillus subtilis. Nature. 1971 Apr 30;230(5296):567–569. doi: 10.1038/230567a0. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Mandelstam J. Sporulation of Bacillus subtilis in continuous culture. J Bacteriol. 1970 Sep;103(3):529–535. doi: 10.1128/jb.103.3.529-535.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Levinson H. S. Fine structure of Bacillus megaterium during microcycle sporogenesis. J Bacteriol. 1967 Aug;94(2):441–457. doi: 10.1128/jb.94.2.441-457.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E. Sporulation of bacilli, a model of cellular differentiation. Curr Top Dev Biol. 1972;7:85–124. doi: 10.1016/s0070-2153(08)60070-8. [DOI] [PubMed] [Google Scholar]
- Greene R. A., Slepecky R. A. Minimal requirements for commitment to sporulation in Bacillus megaterium. J Bacteriol. 1972 Aug;111(2):557–565. doi: 10.1128/jb.111.2.557-565.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HITCHINS A. D., GOULD G. W., HURST A. The swelling of bacterial spores during germination and outgrowth. J Gen Microbiol. 1963 Mar;30:445–453. doi: 10.1099/00221287-30-3-445. [DOI] [PubMed] [Google Scholar]
- Hitchins A. D., Greene R. A., Slepecky R. A. Effect of carbon source on size and associated properties of Bacillus megaterium spores. J Bacteriol. 1972 Apr;110(1):392–401. doi: 10.1128/jb.110.1.392-401.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins A. D., Slepecky R. A. Bacterial sporulation as a modified procaryotic cell division. Nature. 1969 Aug 23;223(5208):804–807. doi: 10.1038/223804a0. [DOI] [PubMed] [Google Scholar]
- Holmes P. K., Levinson H. S. Metabolic requirements for microcycle sporogenesis of Bacillus megaterium. J Bacteriol. 1967 Aug;94(2):434–440. doi: 10.1128/jb.94.2.434-440.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyem T., Rodenberg S., Douthit H. A., Halvorson H. O. Changes in the pattern of proteins synthesized during outgrowth and microcycle in Bacillus cereus T. Arch Biochem Biophys. 1968 Jun;125(3):964–974. doi: 10.1016/0003-9861(68)90535-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MacKechnie I., Hanson R. S. Microcycle sporogenesis of Bacillus cereus in a chemically defined medium. J Bacteriol. 1968 Feb;95(2):355–359. doi: 10.1128/jb.95.2.355-359.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- OISHI M., YOSHIKAWA H., SUEOKA N. SYNCHRONOUS AND DICHOTOMOUS REPLICATIONS OF THE BACILLUS SUBTILIS CHROMOSOME DURING SPORE GERMINATION. Nature. 1964 Dec 12;204:1069–1073. doi: 10.1038/2041069a0. [DOI] [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L. Comparative aspects of morphogenesis in three prokaryotic genera. Annu Rev Microbiol. 1973;27:133–153. doi: 10.1146/annurev.mi.27.100173.001025. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. II. Relationship between ordered enzyme synthesis and deoxyribonucleic acid replication. J Bacteriol. 1968 Feb;95(2):479–489. doi: 10.1128/jb.95.2.479-489.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter V., Slepecky R. A. Direct Transition of Outgrowing Bacterial Spores to New Sporangia Without Intermediate Cell Division. J Bacteriol. 1965 Sep;90(3):803–807. doi: 10.1128/jb.90.3.803-807.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. C., Pardee A. B. Cell division of Escherichia coli: control by membrane organization. J Bacteriol. 1973 May;114(2):603–611. doi: 10.1128/jb.114.2.603-611.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., O'SULLIVAN A., SUEOKA N. SEQUENTIAL REPLICATION OF THE BACILLUS SUBTILIS CHROMOSOME. 3. REGULATION OF INITIATION. Proc Natl Acad Sci U S A. 1964 Oct;52:973–980. doi: 10.1073/pnas.52.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H. DNA synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1476–1483. doi: 10.1073/pnas.53.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]


