Abstract
Background
Intermediate syndrome (IMS) is a major cause of death from respiratory failure following acute organophosphate poisoning. The objective of this study was to determine repetitive nerve stimulation (RNS) predictors of IMS that would assist in patient management and clinical research.
Methods and Findings
Seventy-eight consenting symptomatic patients with organophosphate poisoning were assessed prospectively with daily physical examination and RNS. RNS was done on the right and left median and ulnar nerves at 1, 3, 10, 15, 20, and 30 Hz. The study was conducted as a prospective observational cohort study in the Central Province, Sri Lanka. IMS was diagnosed in ten out of 78 patients using a priori clinical diagnostic criteria, and five of them developed respiratory failure. All ten patients showed progressive RNS changes correlating with the severity of IMS. A decrement-increment was observed at intermediate and high frequencies preceding the onset of clinical signs of IMS. As the patient developed clinical signs of IMS, decrement-increment was progressively noted at low and intermediate frequencies and a combination of decrement-increment and repetitive fade or severe decrement was noted at high frequencies. Severe decrement preceded respiratory failure in four patients. Thirty patients developed forme fruste IMS with less severe weakness not progressing to respiratory failure whose RNS was characterized by decrement-increment or a combination of decrement-increment and repetitive fade but never severe decrements.
Conclusions
Characteristic changes in RNS, preceding the development of IMS, help to identify a subgroup of patients at high risk of developing respiratory failure. The forme fruste IMS with the characteristic early changes on RNS indicates that IMS is a spectrum disorder. RNS changes are objective and precede the diagnosis and complications of IMS. Thus they may be useful in clinical management and research.
Jayawardane and colleagues evaluate a cohort of 78 patients with organophosphate poisoning from Sri Lanka, and identify changes in repetitive nerve stimulation that precede, and may help predict, the onset of intermediate syndrome.
Editors' Summary
Background.
Each year, many thousands of deaths around the world are caused by pesticide poisoning. Often, the pesticide involved is an organophosphate. These highly toxic compounds, which are widely used in agriculture, particularly in developing countries, disrupt the transmission of messages from the brain to the body in insect pests and in people. The brain controls body movements by sending electrical impulses along nerve cells (neurons). At the end of the neurons, these impulses are converted into chemical messengers (neurotransmitters), which cross the gap between neurons and muscle cells (the neuromuscular junction) and bind to proteins on the surface of the muscle cells to pass on the brain's message. One important neurotransmitter is acetylcholine. This is used in the part of the nervous system that controls breathing and other automatic vital functions, at neuromuscular junctions, and in parts of the central nervous system. Normally, acetylcholine is quickly broken down after it has delivered its message, but organophosphates disrupt this process and, consequently, affect nerve transmission to muscles. Organophosphate poisoning causes three syndromes. The cholinergic syndrome, which can be fatal, happens soon after organophosphates are swallowed, inhaled, or absorbed through the skin. The intermediate syndrome (IMS), which results in muscle weakness in the limbs, neck, and throat, develops in some patients 24–96 hours after poisoning. Finally, long-term nerve damage sometimes develops 2–3 weeks after poisoning.
Why Was This Study Done?
Although IMS is a major contributor to the illness caused by organophosphate poisoning and can result in respiratory (breathing) failure and death, the functional changes that are associated with IMS (its pathophysiology) are poorly understood. With a better understanding of these changes, it might be possible to find ways to prevent or treat IMS or to predict which patients with IMS are likely to develop respiratory failure. In this study, the researchers make a set of measurements of nerve transmission in a large group of organophosphate-poisoned patients in Sri Lanka to discover more about the pathophysiology of IMS.
What Did the Researchers Do and Find?
Seventy-eight patients with organophosphate poisoning were assessed several times a day for clinical signs of IMS. In addition, nerve transmission was measured daily in the patients using an electrophysiological technique called repetitive nerve stimulation (RNS). For this, a series of small electrical shocks was applied to the certain nerves in the arm and the responses in the muscles that these nerves control were recorded. In the ten study participants who developed IMS, the researchers observed several characteristic changes in their muscle responses to RNS, some of which were seen before the clinical signs of IMS. Other changes in muscle responses to RNS correlated with the development of clear IMS. Most importantly, in the four patients with IMS who developed respiratory failure, an RNS response pattern called severe decrement (a reduced response to the first electrical shock and then no response to the subsequent shocks) was seen before respiratory failure. Finally, there were other characteristic changes in muscle responses to RNS in 30 patients with muscle weakness not severe enough for a diagnosis of IMS (incomplete or “forme fruste” IMS).
What Do These Findings Mean?
These findings indicate that changes in nerve transmission that can be objectively monitored using RNS evolve during the development of IMS. In other words, IMS is a “spectrum” disorder in which the weakness and neuromuscular junction problems caused by organophosphate poisoning gradually progress over time through a series of electrophysiological changes that will sometimes resolve quickly and only in the most severe cases will result in respiratory failure. These findings need to be validated in further studies, particularly since most of the patients in this study had been exposed to a single organophosphate (chlorpyrifos). However, they suggest that the RNS tests might be useful in the clinical management of patients with organophosphate poisoning, particularly since such tests could provide an early warning of impending respiratory failure.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0050147.
This study is further discussed in a PLoS Medicine Perspective by Cynthia Aaron
The US Environmental Protection Agency provides information about all aspects of pesticides (in English and Spanish)
Toxtown, an interactive site from the US National Library of Science, provides information on environmental health concerns including exposure to pesticides (in English and Spanish)
The US National Pesticide Information Center provides objective, science-based information about pesticides
MedlinePlus also provides links to information on pesticides (in English and Spanish)
The International Programme on Chemical Safety has information on poisoning prevention and management; its INTOX databank has a description of the cholinergic syndrome
Introduction
Organophosphate (OP) poisoning is a major global health problem [1,2] with hundreds of thousands of deaths every year [3,4]. OP poisoning leads to three main syndromes: (1) acute cholinergic syndrome, (2) intermediate syndrome (IMS), and (3) OP-induced delayed polyneuropathy (OPIDPN). IMS remains a major contributor to the high morbidity and mortality in OP poisoning, an important and expensive medical problem in the under-resourced developing world [5].
IMS was first described [5] as a syndrome of muscular paralysis occurring in conscious patients 24–96 h following ingestion, after their acute cholinergic syndrome was treated with atropine. Muscle weakness affected predominantly the proximal limb muscles and those supplied by the cranial nerves. IMS was often associated with respiratory failure. More recent work suggest that IMS could occur before 24 h and even after 96 h [6–8]. The pathophysiology of IMS is not clearly understood [8–15] but is generally believed to result from a persistent excess of acetylcholine (ACh) at the neuromuscular junction [8,11,13,14,16–21].
Neuromuscular transmission has been recorded in OP poisoning in general and in patients with IMS using repetitive nerve stimulation (RNS) and single fiber electromyography [8,22–27]. Neuromuscular transmission failure induced by OPs and other anticholinesterases (e.g., neostigmine) has been extensively studied in animals especially in rat models [23,28–32]. However there are no previous studies to our knowledge that have evaluated patients with serial RNS from the onset of poisoning to describe sequential electrophysiological changes that correlate with clinical severity in IMS. The objective of this study was to serially assess the neuromuscular junctional function with RNS in patients who are at risk of developing IMS to gather more information on the pathophysiology, and to determine any predictors of the syndrome that would be relevant clinically and in research.
Methods
A prospective observational study of symptomatic OP poisoned patients was carried out in the Central Province of Sri Lanka with the approval of the Ethics Committees of the University of Peradeniya, Sri Lanka and the Australian National University, Canberra, Australia. Patients were recruited from Nuwara Eliya General Hospital, Nuwara Eliya from May 2005 to April 2005 and from Teaching Hospital, Peradeniya from May 2006 to December 2006.
All patients were assessed on admission and repeatedly thereafter for features of acute cholinergic syndrome. The inclusion criteria were admission within 24 h of ingestion of OP and signs of systemic intoxication. Patients <15 y of age and pregnant patients were excluded. Informed written consent was obtained from all the study patients. When it was initially not possible to get consent from the patients (e.g., when they were unconscious), consent was obtained from the accompanying relatives but this was later confirmed from the patients themselves when they regained consciousness and were orientated and rational.
OP poisoning was confirmed by the history from the patient and/or relatives, containers brought to hospital, records in patient-transfer forms, characteristic smell in the breath, and clinical features typical of OP poisoning. OP concentration in plasma was quantified in 67 of 78 patients using reversed phase high performance liquid chromatography and ultraviolet detection [33]. Red blood cell acetylcholinesterase (RBC AChE) level was assessed in 59 of 78 patients using modified Elman method [34]. Accordingly, additional biochemical evidence of OP poisoning (serum OP level or RBC AChE level) was available in 69 of the 78 patients.
As per institutional practices patients were treated with 10–15 mg bolus dose of atropine followed by 10–15 mg IV atropine infusion in 0.9% normal saline over about 12 h. The infusion rate was adjusted according to the patient's clinical situation. If the patient became excessively atropinized the infusion was discontinued. If the patient developed further cholinergic features further boluses (e.g., 10–15 mg IV) were given.
Pralidoxime was administered 1 g q 6 h for 48 h as a slow IV injection.
Clinical and Electrophysiological Assessment of Patients
All the patients were assessed at least twice daily with a focused neurological examination to detect signs of IMS, and at least six times a day for cholinergic signs. Bedside electrophysiological testing was carried out using a portable Medelec Synergy electromyography machine (software version 11). The first assessment was done within 24 h of poisoning in 69 of 78 patients. The studies were repeated daily until there were no detectable electrophysiological or clinical abnormalities.
RNS was performed on right and left median and ulnar nerves. Single supramaximal stimulation of the same nerves was done to detect repetitive responses. Nerves were stimulated superficially by a stimulator fixed to the respective nerves at the wrist. Recordings were done with TECA NCS disposable bar electrodes using the belly tendon configuration. The “stimulation” hand was immobilized manually to prevent movement artifacts. A 50-Hz notch filter was used. RNS studies were done with train of ten supramaximal stimuli of 0.1 ms at 1, 3, 10, 15, 20, and 30 Hz frequencies. There was at least a 15 s interval between two trains of stimuli.
To confirm that motor conduction velocity was unaffected, we measured this parameter in the first 60 patients. Motor conduction velocity was normal despite clinical weakness. This confirmed previous findings reported by others [5,26,35,36] and we thus did not formally measure motor conduction velocities in further patients.
A prospective definition for IMS was developed on the basis of Senanayake and Karalliedde's original description [5], namely significant muscle weakness in at least three of the following muscle groups (extraocular, neck flexor, proximal limb, and facial) observed at least 24 h after ingestion of OP. The weakness of proximal muscles and neck flexion was considered significant when the muscle power was grade 3 or less (Medical Research Council [MRC] grading): weakness of respiratory muscles was not considered a requirement for the diagnosis. While cholinergic features had usually subsided at this time, short relapses of muscarinic signs or symptoms would not exclude the diagnosis.
Data are presented with conventional nonparametric descriptive summary statistics.
Results
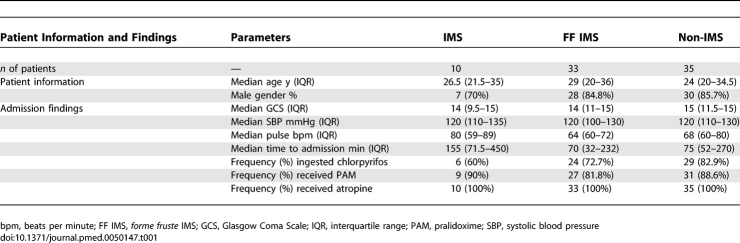
Of a total of 91 patients recruited, serial clinical and electrophysiological assessments could be successfully completed in 78 (65 males). In 13 patients serial electrophysiological studies were not successful (withdrawal of consent, four; unable to perform due to restlessness/delirium, seven; technical difficulties, two). The OP ingested was chlorpyrifos in 59 of 78 patients. Two patients have ingested dimethoate:1 phenthoate:1 diazinon. In four patients, either the OP was not detected (n = 2), or the type of OP was not confirmed. Blood samples were not available or were not assayed for OP levels in 11 patients. Depression of RBC AChE to <50% of the lower limit of the normal range and/or toxicologically significant concentrations of OP was detected in 69 of 78.
IMS was diagnosed in nine of the 78 patients according to our clinical diagnostic criteria. One additional patient developed typical clinical features and characteristic electrophysiological features of IMS 15 h after the ingestion during the cholinergic crisis (increased secretions, pin point pupils, diarrhea, and generalized fasciculations) despite atropine therapy. She was intubated approximately 25 h postingestion and was ventilated for 18 d. Late onset (>24 h) respiratory failure requiring supported ventilation developed in five of the ten IMS patients. Marked weakness of neck flexion and proximal limb muscles (MRC grade 3 or less) was the most consistent feature in all ten patients. The most frequent motor cranial nerve deficits were facial muscle weakness (n = 10) and ptosis (n = 10). In addition, external ophthalmoparesis was observed in one, and dysphagia in another. The onset of muscle weakness varied from 15 h to 6 d. Deep tendon reflexes were absent or depressed in six patients.
Of the ten IMS patients, two had both muscarinic features and fasciculations at the time of diagnosis of IMS. One patient had muscarinic features but no fasciculations, and three of ten patients had fasciculations without any muscarinic signs at the time of diagnosis of IMS. Those who developed respiratory failure took a longer time to recover from the muscle weakness, whilst the others recovered within 24–48 h.
Pattern of RNS Abnormalities Observed in IMS Patients
In five of ten IMS patients (50%) the initial electrophysiological changes were detected within the first 24 h of poisoning and in two patients within 24 and 48 h of poisoning. The sequential changes were as outlined in the following paragraphs.
Decrement-Increment Pattern
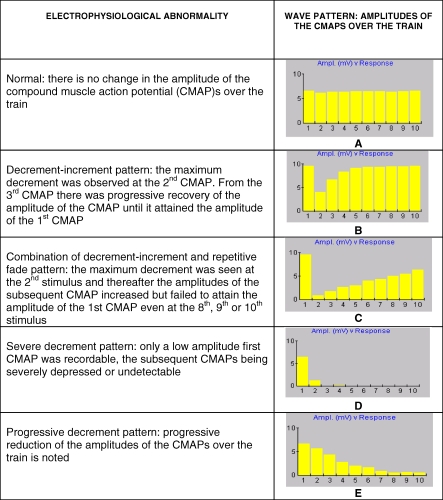
In this pattern, the maximum decrement was observed in the second compound muscle action potential (CMAP). From the third CMAP there was progressive recovery of the amplitude until it attained the amplitude of the first CMAP typically by the fourth, fifth, or sixth (Figures 1B and 2A, 20–30 Hz). Decrement-increment initially occurred in high frequency stimulations (20 and 30 Hz). As the patient deteriorated clinically, the abnormalities occurred progressively at intermediate frequencies (10 and 15 Hz) and then at low frequencies (1 and 3 Hz). The decrement-increment seen at high frequency stimulations changed to a combination of decrement-increment and repetitive fade.
Figure 1. Electrophysiological Abnormalities during the Progression of IMS.
(A) Normal response to train of ten stimuli.
(B) Decrement-increment pattern.
(C) Combination of decrement-increment and repetitive fade pattern.
(D) Severe decrement pattern.
(E) Progressive decrement pattern.
Figure 2. Sequential RNS Changes in IMS.
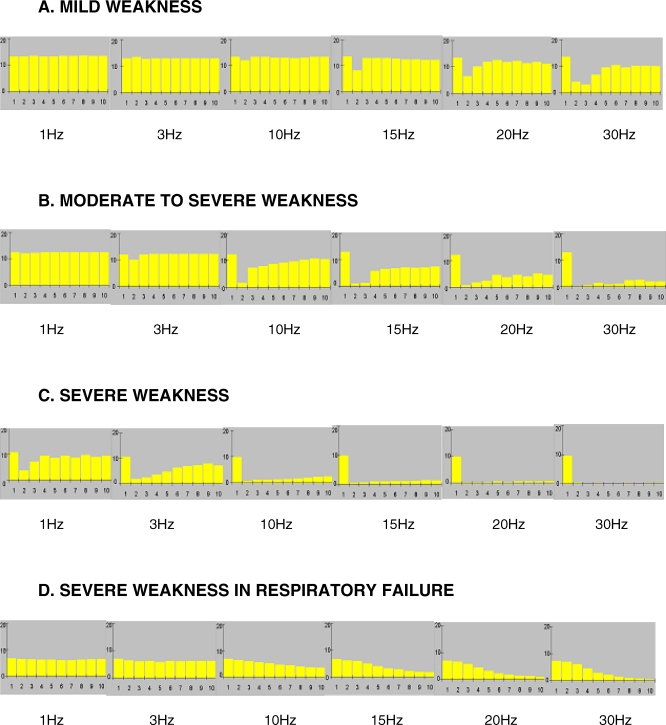
(A–C) Changes in amplitudes of the CMAPs over the train of ten stimuli at varying frequencies in a typical IMS patient (022/OP/NE) as he developed progressively increasing weakness leading to respiratory failure.
(D) RNS abnormalities in a patient who was in respiratory failure (001/OP/PE) following severe muscle weakness.
(A) 20–30 Hz decrement-increment pattern; (B) 15–30 Hz combination of decrement-increment with repetitive fade; (C) 20–30 Hz severe decrement pattern; (D) 10–30 Hz progressive decrement pattern.
Scale y-axis: 0–20 mV
Combination of Decrement-Increment and Repetitive Fade
Maximum decrement was seen at the second stimulus and thereafter the amplitudes of the subsequent CMAP increased but failed to attain the amplitude of the first CMAP even at the eight, ninth, or tenth (Figures 1C and 2B, 15–30 Hz) [24]. This pattern was initially seen only at high frequency stimulations. As the muscular weakness progressed, the change occurred at intermediate frequencies and there was a corresponding decrement in the amplitude of the tenth CMAP, which was most marked with high frequency stimulations. Three of the ten IMS patients did not progress beyond combination of decrement-increment and repetitive fade electrophysiologically.
Severe Decrement Pattern
Here only a low amplitude first CMAP was recordable, the subsequent CMAPs being severely depressed or undetectable (Figures 1D and 2C, 30 Hz). This pattern was typically seen at 30 Hz stimulations when patients showed maximal muscle weakness (n = 7). Severe decrement pattern at high frequency stimulations preceded respiratory failure in four patients.
When this pattern was seen at high frequency stimulations, intermediate frequency stimulations usually demonstrated a combination of decrement-increment and repetitive fade phenomena, and at low frequency stimulations, decrement-increment.
Progressive Decrement Pattern
The patients who developed respiratory failure with severe muscle weakness developed progressive decrement following a severe decrement (Figures 1E and 2D). As demonstrated in Figure 2C and 2D, there was a transition of electrophysiological features from severe decrement pattern to progressive decrement pattern.
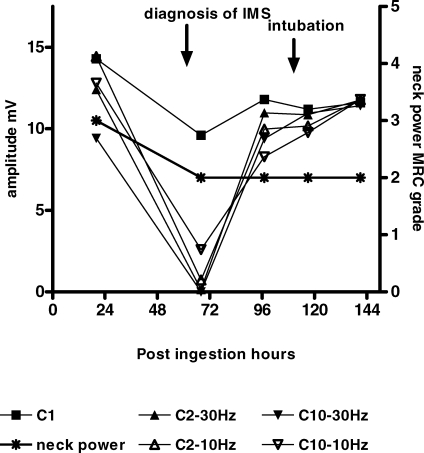
Figure 3 demonstrates how these changes look when plotted longitudinally in a patient who developed respiratory failure following IMS.
Figure 3. Comparison of RNS Changes with Neck Power.
The progressive changes of amplitudes of C1 (first CMAP), C2 (second CMAP), and C10 (tenth CMAP) at 10 Hz and 30 Hz over time in an IMS patient (063/OP/NE) who subsequently developed respiratory failure. Change of neck muscle power is also demonstrated.
Patterns of RNS during the Recovery Stage of IMS
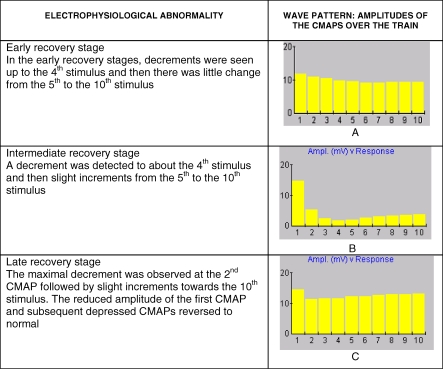
Recovery of RNS features was usually rapid and often preceded clinical improvement in severe cases (see Figure 3 for very marked example of this phenomena). However, in a few IMS patients who developed respiratory failure we observed a particular pattern of RNS abnormalities during recovery rather than a simple reversal of effects. The initial changes during recovery were seen in lower and intermediate frequency stimulations. The last to recover were the high frequency response patterns. In the early recovery stages, decrements were seen up to the fourth stimulus and then there was little change from the fifth to the tenth stimulus (Figure 4A). Following this, a decrement was detected to about the fourth stimulus and then slight increments from the fifth to the tenth stimulus (Figure 4B) Subsequently, the pattern changed where the maximal decrement was observed at the second CMAP followed by slight increments towards the tenth stimulus (Figure 4C). The reduced amplitude of the first CMAP and subsequent depressed CMAPs reversed to normal (Figure 1A) but with significant interpatient variation.
Figure 4. Patterns of RNS during the Recovery Period of IMS.
(A) RNS abnormalities seen during the early recovery stage, (B) RNS abnormalities seen during intermediate stage of recovery, and (C) RNS abnormalities at the late recovery stage.
Those patients who did not develop respiratory failure but showed severe decrement or a combination of decrement-increment and repetitive fade recovered earlier than those who developed respiratory failure. The RNS abnormalities normalized within the next 24 h of the observation of electrophysiological abnormalities.
Observation of a “forme fruste” IMS
A group of patients (30/78) developed varying degrees of weakness, involving the neck flexors, proximal limb muscles, and muscles supplied by the motor cranial nerves, and accompanied by characteristic electrophysiological abnormalities identical to early or intermediate stage changes that were seen in IMS patients. We considered this a “forme fruste IMS,” because they did not meet our study criteria for a diagnosis of classical IMS.
Of these 30 patients five developed weakness of the neck flexors, proximal limb muscles, and cranial nerves but did not meet predefined criteria for a clinical diagnosis of IMS as they had only mild (MRC grade 4) weakness. Three of the five patients developed a combination of decrement-increment and repetitive fade and two of five patients developed only decrement-increment.
Twenty-five of 30 developed weakness of one or two of the three muscle groups, but not all three. Of those, seven patients developed decrement-increment and a combination of decrement-increment and repetitive fade and 18 developed decrement-increments only. None of the 30 patients who developed the forme fruste IMS showed severe decrement on RNS.
Twenty-one of 30 (70%) developed electrophysiological abnormalities within 48 h of ingestion of the poison. These patients showed rapid clinical recovery, which was accompanied by normal electrophysiological recordings.
There were three of 78 patients who did not develop clinically detectable muscle weakness but developed decrement-increment at high frequency stimulations.
Those who were not diagnosed as IMS or forme fruste IMS, did not develop decrement-increment patterns or severe decrement patterns on serial RNS studies. They had normal RNS and at high frequency stimulations pseudo-facilitation was noted.
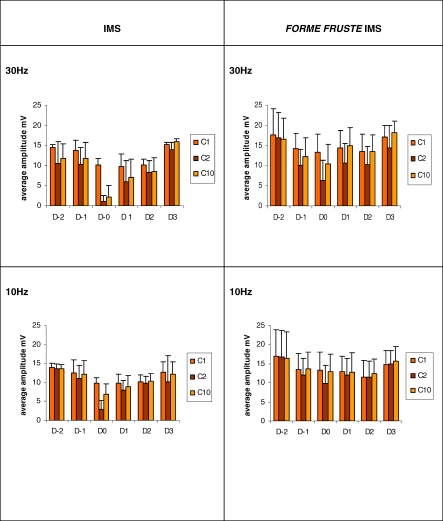
The average amplitudes of C1 (first CMAP), C2 (second CMAP), and C10 (tenth CMAP) at 10Hz and 30Hz over varying stages of the illness in the classical IMS and the forme fruste IMS were seen to be different (Figure 5).
Figure 5. Comparison of Average Amplitudes of C1, C2, and C10 between IMS and forme fruste Groups.
The average amplitude of C1 (first CMAP), C2 (second CMAP), and C10 (tenth CMAP) in the Clinical IMS and forme fruste IMS at 10Hz and 30Hz frequency. Changes are plotted for each day relative to day 0 (D0). D0 in the clinical IMS group is the day they were diagnosed as clinical IMS. The D0 in forme fruste group is the day they were detected to have maximal muscle weakness.
There was no difference in the treatment regimes between those who developed IMS spectrum disorder and those who did not. Both groups received 10–15 mg of a bolus dose of intravenous atropine followed by 10–15 mg atropine infusion over 12–24 h. The rate was adjusted according to the patients' cholinergic status. Sixty-seven of 78 patients were treated with pralidoxime 1g q 6 h for 48 h as per institutional practice. Eleven (one IMS patient, six forme fruste IMS patients, and four non-IMS patients) were not treated with pralidoxime as the drug was not available.
Refer to Table 1 for presenting disease severity, admission characteristics, and the treatment details of IMS, forme fruste IMS, and other patients.
Table 1.
Presenting Disease Severity, Admission Characteristics, and the Treatment Details of IMS, forme fruste IMS, and Other Patients
Discussion
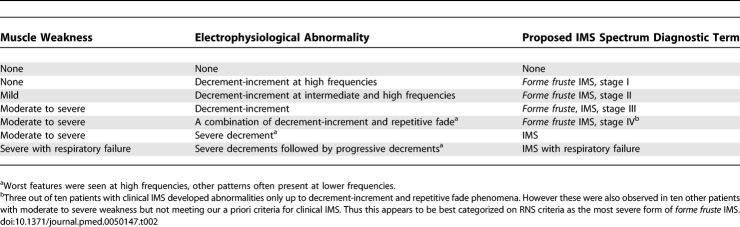
We found characteristic abnormalities on RNS associated with the development and resolution of muscle weakness in IMS. These clinical and electrophysiological changes varied in severity, presumably representing a continuum through which patients progress over time (Table 2).
Table 2.
Proposed Criteria for Intermediate Spectrum Disorder Diagnosis
The degree of muscle weakness correlated well with the electrophysiological features during progression of IMS. The only time there appeared to be a break in the continuum was when the patients developed respiratory muscle weakness; then there was a transition of the electrophysiological features from decrement-increment and severe decrement to progressive decrement. This occurred only in the group who fulfilled our a priori diagnostic criteria, suggesting that these criteria are clinically useful in identifying patients at risk of late respiratory failure.
Although the electrophysiological features closely parallel the clinical severity during progression of IMS, the same was not true during recovery. Electrophysiological changes sometimes improved long before the patient recovered normal strength and respiratory efforts (Figure 3). This confirms in a much larger cohort, the observation reported by de Bleecker in 1993 that the electromyography usually normalized before the neurological symptoms [11]. However, in both these studies only the distal muscles were measured, and these are the least clinically affected in IMS. Phrenic nerve conduction studies done by Singh et al. showed reduced CMAP amplitude of diaphragmatic muscle correlated well with the need for mechanical ventilation in OP poisoned patients. Further, normalization of the diaphragmatic CMAP amplitudes was noted in patients who could be successfully weaned, and persistent or rebound abnormalities in those who failed a trial of ventilatory discontinuation [26].
Some of these electrophysiological features have been described earlier [8,22–26,35,37]. Animal studies have been done to assess the nature of the neuromuscular transmission failure that occurs following anticholinesterase/OP poisoning. In 1991 Maselli et al. studied rats injected with diisopropylfluorophosphate (DFP). They noted decrement-increment that was accentuated at high frequency stimulations. Edrophonium accentuated the decrement response; in contrast D-tubocurarine corrected the electrophysiological decrement [23]. In 1992 Besser et al. studied left phrenic hemi-diaphragm preparations from rats. AChE was inhibited by a transient perfusion of the hemi-diaphragm with neostigmine. They observed decrement-increment and in some trains the second CMAP was abolished without subsequent recovery by the ninth stimulus [31]. This is similar to the severe decrements we observed in OP-poisoned patients who had severe muscle weakness. De Bleecker et al. studied rats poisoned with paraoxon and fenthion (800 mg/kg subcutaneous). At high frequency stimulations they observed the two types of decrements (decrement-increment phenomena and decrement phenomena). The decrement-increment phenomenon preceded the decrement phenomenon and was associated with slightly less severe AChE inhibition than decrement [28].
This is the largest prospective cohort study evaluating the electrophysiological abnormalities in IMS and the first study that has evaluated patients with serial RNS from the onset of poisoning to describe sequential electrophysiological changes that correlate with progression of the clinical severity in IMS. An advantage of this study is the inclusion of a group of symptomatic OP poisoned patients who did not demonstrate electrophysiological evidence of neuromuscular transmission failure.
These findings clearly demonstrate that IMS is a spectrum disorder. At one end of the spectrum the patients demonstrate only the electrophysiological abnormalities without clinically detectable muscle weakness, and at the other end, patients progress to severe muscular weakness with deterioration of electrophysiological measurements and the risk of respiratory failure. While our a priori diagnostic criteria restricted a diagnosis of IMS to patients with MRC weakness of grade 3 or less, this did identify a subgroup with worse prognosis. Typical RNS findings in the forme fruste group suggest that weakness of greater than MRC grade 3 in neck flexors and proximal limb muscles with or without weakness of muscles supplied by the motor cranial nerves is clinically useful as it reveals a much higher incidence of IMS than that is usually described. Further we propose objective criteria for the diagnosis of the stage of the IMS spectrum disorder on the basis of our clinical and electrophysiological observations (Table 2). These criteria need to be validated in future prospective studies, however if adopted, they may reduce the equivocation caused by the widespread nonspecific use of the term IMS.
The original description of IMS states that it occurs distinctly separate from the cholinergic syndrome. However in the present study one patient was acutely cholinergic while being diagnosed as IMS and some had short relapses of muscarinic signs at the diagnosis of IMS. The electrophysiological profiles of these patients were not different from the others without overt muscarinic signs. This confirms what was observed previously by De Bleecker et al. in 1993 [11].
Oximes may potentially affect on the development of IMS [12,38,39]. Addressing this issue is beyond the scope of this study, as only a few patients did not receive pralidoxime and our patients only received intermittent low dose pralidoxime for approximately 48 h. However, there is a need for further prospective studies with serial electrophysiological monitoring to measure the effects of pralidoxime on the neuromuscular junction and the development of IMS.
The early detection of RNS changes in IMS suggests that underlying mechanism or mechanisms occur early. The key stage where neuromuscular junction transmission fails even to support respiratory function coincides with an electrophysiological transition. The change from decrement-increment/severe decrements to progressive decrement is a feature that has not been described before, and it may point to a specific pathological process at this time.
The transient depolarization of the end-plate receptor is the main factor accounting for the failure of neuromuscular transmission observed during the exposure of the rat neuromuscular junction to low concentration of acetylcholinesterase [23]. In this study forme fruste IMS patients and classical IMS patients, exhibited the decrement-increment pattern during early stage of intoxication. In classical IMS patients, decrement-increment pattern worsened to severe decrement pattern at high frequency stimulations. Thus it is possible that at early stages or in less severe cases there is depolarization block at the neuromuscular junction. Persistent accumulation of acetylcholine at the neuromuscular junction may lead to desensitization block as described by Maselli et al. in 1993 [30]. Desensitization is said to counteract the depolarization block but in the presence of high concentrations of anticholinesterase, desensitization intensifies the blockade and it becomes the dominant mechanism of neuromuscular transmission failure. The transition of electrophysiological abnormalities from severe decrement to progressive decrement pattern observed in our study may represent the transition between the two types of neuromuscular blockade. It has also been suggested that the persistent accumulation of ACh at the nicotinic receptor causes either down regulation [40] or conformational change of the N-receptor [15]. That could lead to a reduction of the number of functioning N-receptors at the postsynaptic junction. Further the direct effect of OP at the neuromuscular junction causing conformational change as suggested by Katz et al. [15] cannot be ruled out. This would be one possible explanation for why some OPs have been demonstrated in both animals and humans to be associated with a much higher incidence of IMS [28,33].
The electrophysiological findings in other common neuromuscular junction disorders suggest that, the electrophysiological findings of the IMS spectrum disorder are unlikely to represent a single simple pre- or postsynaptic defect. Myasthenia gravis causes a reduced density of functioning AChR at the postjunctional membrane [41]. The characteristic response to RNS at 2 to 3 Hz stimulation rate in myasthenia gravis is a progressive decrement in amplitude of the CMAP, which is most reliably found in weak muscles [42]. In Lambert-Eaton syndrome, there is a presynaptic neuronal conduction block. Low amplitude CMAPs are seen on motor nerve stimulation with marked facilitation of CMAP amplitude after brief muscle contraction or following high-frequency (20–50 Hz) RNS [43,44]. In botulism there is enzymatic cleavage of proteins that are needed for the exocytosis of ACh. As a result, ACh cannot be released and the muscle is paralyzed [45]. The most consistent electrophysiological abnormality is a small evoked muscle action potential in response to a single supramaximal nerve stimulus in a clinically affected muscle. A decrement response to RNS at slow rates (2–3 Hz) is also occasionally seen [45]. Congenital end-plate acetylcholinesterase deficiency produces decrement patterns in RNS studies. Kohara et al. reported a case in which they have noticed decrement-increment pattern at 20 Hz stimulation in mildly affected muscle [46]. However, the cause of weakness and RNS findings in this rare condition are not well understood but postulated to be explained by both presynaptic (e.g., decreased choline substrate for ACh production, negative feedback, damage to nerve terminal) and postsynaptic (e.g., end-plate damage or persistent partial depolarization, receptor desensitization) responses to persistently high levels of ACh [47].
It seems likely that the pathogenesis of IMS in man is complex. The toxicokinetics and dynamics of each specific OP and the treatment the patient receives may influence whether or not the condition develops. Inadequate treatment with oximes may play a role, especially with di-ethyl OP that should respond well [33]. It is also possible that there may be a genetic susceptibility with some patients having altered ACh pathways at the neuromuscular junction and postsynaptic nicotinic receptor polymorphisms. Animal models of IMS would be helpful to fully explore each of these potential sources of variability.
Our electrophysiological findings could be used for objective clinical assessments in the management of patients following OP poisoning and in research (such as evaluating effects of therapeutic agents or therapeutic regimen) once they are validated in future prospective studies. Sequential RNS need to be carried out to detect the development of the severe decrement pattern, an ominous sign indicating that respiratory failure is imminent. Less severe but evolving abnormalities may indicate the need for longer observation or transfer to higher level of care.
Conclusions
Our findings suggest that IMS is a spectrum disorder in which the initial pathological process starts with acute cholinergic state, which may or may not progress through a series of electrophysiological changes leading to respiratory failure and progressive decrement on RNS. Evolving electrophysiological findings provide valuable new objective information to facilitate assessments and to predict the development of IMS. While these need to be validated in future prospective studies, this study also provides objective clinical/electrophysiological criteria for different stages of the IMS spectrum. It is intended that these findings will stimulate further clinical studies to determine the pathogenesis of IMS, which may in turn help to improve the management of OP poisoning.
Supporting Information
(95 KB DOC)
Acknowledgments
We thank Yamuna Dissanayake, S. Samarakoon, and Keerthi Kularatne, the consultant physicians of Nuwara Eliya General Hospital and Teaching Hospital Peradeniya, who were responsible for the medical care of the study patients; medical superintendent, nursing, and medical staff of the same for their immense support; and Nuwara Eliya SACTRC poison unit research team for their invaluable work. Peter Eyer, Horst Thiermann, L. Szinicz, and Franz Worek of Munich are gratefully acknowledged for analysis of the blood samples. We thank Kent Olson and Michael Eddleston for their critical review.
Abbreviations
- ACh
acetylcholine
- CMAP
compound muscle action potential
- IMS
intermediate syndrome
- MRC
Medical Research Council
- OP
organophosphate
- RBC AChE
red blood cell acetylcholinesterase
- RNS
repetitive nerve stimulation
Footnotes
Author contributions. PJ participated in the design and planning of the study, drafted the study proposal, collected patient information, carried out the electrophysiological studies and clinical examinations, analyzed and interpreted data, interpreted evidence, wrote the first draft, and took part in revising and finalizing the paper. PJ had full access to all the data in the study and had final responsibility for the decision to submit for publication. AHD participated in the design and planning of the study, edited and revised the study proposal, supervised the study, interpreted results, interpreted evidence, edited the paper, and took part in revising the paper and approved the final version. VW participated in the design and planning of the study, edited and revised the study proposal, supervised the study, and approved the final version. LK interpreted evidence, edited the paper, and took part in revising the paper and approved the final version. NAB participated in the design and planning of the study, interpreted evidence, edited the paper, and took part in revising the paper and approved the final version. NS participated in the design and planning of the study, edited and revised the study proposal, supervised the study, interpreted results, interpreted evidence, edited the paper, and took part in revising the paper and approved the final version.
Funding: This study was supported by the South Asian Clinical Toxicology Research Collaboration, which is funded by the Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant GR071669MA. The study sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing Interests: The authors have declared that no competing interests exist.
References
- Karalliedde L, Senanayake N. Acute organophosphorus insecticide poisoning in Sri Lanka. Forensic Sci Int. 1988;36:97–100. doi: 10.1016/0379-0738(88)90220-4. [DOI] [PubMed] [Google Scholar]
- Langley R, Sumner D. Pesticide mortality in the United States 1979–1998. Vet Hum Toxicol. 2002;44:101–105. [PubMed] [Google Scholar]
- Buckley NA, Roberts D, Eddleston M. Overcoming apathy in research on organophosphate poisoning. BMJ. 2004;329:1231–1233. doi: 10.1136/bmj.329.7476.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NA, Karalliedde L, Dawson A, Senanayake N, Eddleston M. Where is the evidence for treatments used in pesticide poisoning? Is clinical toxicology fiddling while the developing world burns. J Toxicol Clin Toxicol. 2004;42:113–116. doi: 10.1081/clt-120028756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med. 1987;316:761–763. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Mohamed F, Davies JO, Eyer P, Worek F, et al. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. 2006;99:513–522. doi: 10.1093/qjmed/hcl065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xu H, Qin F, Xu L, Huang J, et al. Intermediate myasthenia syndrome following acute organophosphates poisoning–an analysis of 21 cases. Hum Exp Toxicol. 1998;17:40–45. doi: 10.1177/096032719801700107. [DOI] [PubMed] [Google Scholar]
- Avasthi G, Singh G. Serial neuro-electrophysiological studies in acute organophosphate poisoning:correlation with clinical findings, serum cholinesterase levels and atropine dosages. J Assoc Physicians India. 2000;48:794–799. [PubMed] [Google Scholar]
- Sedgwick EM, Senanayake N. Pathophysiology of the intermediate syndrome of organophosphorus poisoning. J Neurol Neurosurg Psychiatry. 1997;62:201–202. doi: 10.1136/jnnp.62.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalliedde L, Henry JA. Effects of organophosphates on skeletal muscle. Hum Exp Toxicol. 1993;12:289–296. doi: 10.1177/096032719301200406. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Van den Neucker K, Colardyn F. Intermediate syndrome in organophosphorus poisoning: a prospective study. Crit Care Med. 1993;21:1706–1711. doi: 10.1097/00003246-199311000-00020. [DOI] [PubMed] [Google Scholar]
- Benson B, Tolo D, Mclntire M. Is intermediate syndrome in organophosphate poisoning the result of insufficient oxime therapy. J Toxicol Clin Toxicol. 1992;30:347–349. [Google Scholar]
- John M, Oommen A, Zachariah A. Muscle injury in organophosphorous poisoning and its role in the development of intermediate syndrome. Neurotoxicology. 2003;24:43–53. doi: 10.1016/s0161-813x(02)00111-0. [DOI] [PubMed] [Google Scholar]
- Dandapani M, Zachariah A, Kavitha MR, Jeyaseelan L, Oommen A. Oxidative damage in intermediate syndrome of acute organophosphorous poisoning. Indian J Med Res. 2003;117:253–259. [PubMed] [Google Scholar]
- Katz EJ, Cortes IV, Eldefrawi EM, Eldefrawi AT. Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylecholine receptor: relevance to their toxicities. Toxicol Appl Pharmacol. 1997;146:227–236. doi: 10.1006/taap.1997.8201. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Willems J, Van Den Neucker K, De Reuck J, Vogelaers D. Prolonged toxicity with intermediate syndrome after combined parathion and methyl parathion poisoning. J Toxicol Clin Toxicol. 1992;30:333–345. 347–349. doi: 10.3109/15563659209021548. discussion. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Van Den Neucker K, Willems J. The intermediate syndrome in organophosphate poisoning: presentation of a case and review of the literature. J Toxicol Clin Toxicol. 1992;30:321–329. 331–332. doi: 10.3109/15563659209021546. discussion. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Volgelaers D, Ceutericl C, Van Den Neucker K, Williems J, et al. Intermediate syndrome due to prolonged parathion poisoning. Acta Neurologica Scand. 1992;86:421–424. doi: 10.1111/j.1600-0404.1992.tb05110.x. [DOI] [PubMed] [Google Scholar]
- De Bleecker JL. Intermediate syndrome: prolonged cholinesterase inhibition. J Toxicol Clin Toxicol. 1993;31:197–199. doi: 10.3109/15563659309000385. [DOI] [PubMed] [Google Scholar]
- De Bleecker JL. Multiple system organ failure: link to intermediate syndrome indirect. J Toxicol Clin Toxicol. 1996;34:249–250. doi: 10.3109/15563659609013781. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Lison D, Van Den Abeele K, Willems J, De Reuck J. Acute and subacute organophosphate poisoning in the rat. Neurotoxicology. 1994;15:341–348. [PubMed] [Google Scholar]
- Besser R, Gutman L, Weilemann LS. Inactivation of end-plate acetylcholinesterase during the course of organophosphate intoxications. Arch Toxicol. 1989;63:412–415. doi: 10.1007/BF00303132. [DOI] [PubMed] [Google Scholar]
- Maselli RA, Soliven BC. Analysis of the organophosphate-induced electromyographic response to repetitive nerve stimulation: paradoxical response to edrophonium and D-tubocurarine. Muscle Nerve. 1991;14:1182–1188. doi: 10.1002/mus.880141207. [DOI] [PubMed] [Google Scholar]
- Besser R, Gutmann L, Dillmann U, Weilemann LS, Hopf HC. End-plate dysfunction in acute organophosphate intoxication. Neurology. 1989;39:561–567. doi: 10.1212/wnl.39.4.561. [DOI] [PubMed] [Google Scholar]
- Besser R, Gutmann L. A quantitative study of the pancuronium antagonism at the motor endplate in human organophosphorus intoxication. Muscle Nerve. 1995;18:956–960. doi: 10.1002/mus.880180906. [DOI] [PubMed] [Google Scholar]
- Singh G, Sidhu UP, Mahajan R, Avasthi G, Whig J. Phrenic nerve conduction studies in acute organophosphate poisoning. Muscle Nerve. 2000;23:627–632. doi: 10.1002/(sici)1097-4598(200004)23:4<627::aid-mus23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Besser R, Vogt T, Gutmann L. Pancuronium improves the neuromuscular transmission defect of human organophosphate intoxication. Neurology. 1990;40:1275–1277. doi: 10.1212/wnl.40.8.1275. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Van den Abeele K, De Reuck J. Electromyography in relation to end-plate acetylcholinesterase in rats poisoned by different organophosphates. Neurotoxicology. 1994;15:331–340. [PubMed] [Google Scholar]
- Dongren Y, Tao L, Fengsheng H. Electroneurophysiological studies in rats of acute dimethoate poisoning. Toxicol Lett. 1999;107:249–254. doi: 10.1016/s0378-4274(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Maselli RA, Leung C. Analysis of anticholinesterase-induced neuromuscular transmission failure. Muscle Nerve. 1993;16:548–553. doi: 10.1002/mus.880160518. [DOI] [PubMed] [Google Scholar]
- Besser R, Vogt T, Gutmann L, Hopf HC, Wessler I. Impaired neuromuscular transmission during partial inhibition of acetylcholinesterase: the role of stimulus-induced antidromic backfiring in the generation of the decrement-increment phenomenon. Muscle Nerve. 1992;15:1072–1080. doi: 10.1002/mus.880151003. [DOI] [PubMed] [Google Scholar]
- Maselli RA, Leung C. Analysis of neuromuscular transmission failure induced by anticholinesterases. Ann N Y Acad Sci. 1993;681:402–404. doi: 10.1111/j.1749-6632.1993.tb22920.x. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288:73–90. doi: 10.1016/s0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]
- Maselli R, Jacobsen JH, Spire JP. Edrophonium: an aid in the diagnosis of acute organophosphate poisoning. Ann Neurol. 1986;19:508–510. doi: 10.1002/ana.410190517. [DOI] [PubMed] [Google Scholar]
- Van den Neucker K, Vanderstraeten G, De Muynck M, De Wilde V. The neurophysiologic examination in organophosphate ester poisoning. Case report and review of the literature. Electromyogr Clin Neurophysiol. 1991;31:507–511. [PubMed] [Google Scholar]
- Poojara L, Vasudevan D, Arun Kumar A, Kamat V. Organophosphate poisoning: diagnosis of Intermediate syndrome. Indian J Crit Care. 2003;7:94–102. [Google Scholar]
- Sudakin DL, Mullins ME, Horowitz BZ, Abshier V, Letzig L. Intermediate syndrome after malathion ingestion despite continuous infusion of pralidoxime. J Toxicol Clin Toxicol. 2000;38:47–50. doi: 10.1081/clt-100100915. [DOI] [PubMed] [Google Scholar]
- de Silva HJ, Wijewickrema R, Senanayake N. Does pralidoxime affect outcome of management in acute organophosphorus poisoning. Lancet. 1992;339:1136–1138. doi: 10.1016/0140-6736(92)90733-j. [DOI] [PubMed] [Google Scholar]
- Karalliedde L, Baker D, Marrs TC. Organophosphate-induced intermediate syndrome: aetiology and relationships with myopathy. Toxicol Rev. 2006;25:1–14. doi: 10.2165/00139709-200625010-00001. [DOI] [PubMed] [Google Scholar]
- Martyn JA, White DA, Gronert GA, Jaffe RS, Ward JM. Up-and-down regulation of skeletal muscle acetylcholine receptors. Effects on neuromuscular blockers. Anesthesiology. 1992;76:822–843. doi: 10.1097/00000542-199205000-00022. [DOI] [PubMed] [Google Scholar]
- Mahadeva B, Phillips LH, 2nd, Juel VC. Autoimmune disorders of neuromuscular transmission. Semin Neurol. 2008;28:212–227. doi: 10.1055/s-2008-1062260. [DOI] [PubMed] [Google Scholar]
- Eaton LM, Lambert EH. Electromyography and electric stimulation of nerves in diseases of motor unit; observations on myasthenic syndrome associated with malignant tumors. J Am Med Assoc. 1957;163:1117–1124. doi: 10.1001/jama.1957.02970480021005. [DOI] [PubMed] [Google Scholar]
- Lambert EH. Defects of neuromuscular transmission in syndromes other than myasthenia gravis. Ann N Y Acad Sci. 1966;135:367–384. doi: 10.1111/j.1749-6632.1966.tb45484.x. [DOI] [PubMed] [Google Scholar]
- Cherington M. Botulism: update and review. Semin Neurol. 2004;24:155–163. doi: 10.1055/s-2004-830901. [DOI] [PubMed] [Google Scholar]
- Kohara N, Lin TS, Fukudome T, Kimura J, Sakamoto T, et al. Pathophysiology of weakness in a patient with congenital end-plate acetylcholinesterase deficiency. Muscle Nerve. 2002;25:585–592. doi: 10.1002/mus.10073. [DOI] [PubMed] [Google Scholar]
- Hutchinson DO, Walls TJ, Nakano S, Camp S, Taylor P, et al. Congenital endplate acetylcholinesterase deficiency. Brain. 1993;116(Pt 3):633–653. doi: 10.1093/brain/116.3.633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(95 KB DOC)