Abstract
The evolution of the complex and dynamic behavioural interactions between caring parents and their dependent offspring is a major area of research in behavioural ecology and quantitative genetics. While behavioural ecologists examine the evolution of interactions between parents and offspring in the light of parent–offspring conflict and its resolution, quantitative geneticists explore the evolution of such interactions in the light of parent–offspring co-adaptation due to combined effects of parental and offspring behaviours on fitness. To date, there is little interaction or integration between these two fields. Here, we first review the merits and limitations of each of these two approaches and show that they provide important complementary insights into the evolution of strategies for offspring begging and parental resource provisioning. We then outline how central ideas from behavioural ecology and quantitative genetics can be combined within a framework based on the concept of behavioural reaction norms, which provides a common basis for behavioural ecologists and quantitative geneticists to study the evolution of parent–offspring interactions. Finally, we discuss how the behavioural reaction norm approach can be used to advance our understanding of parent–offspring conflict by combining information about the genetic basis of traits from quantitative genetics with key insights regarding the adaptive function and dynamic nature of parental and offspring behaviours from behavioural ecology.
Keywords: offspring begging, parent–offspring co-adaptation, parent–offspring conflict, parental care, reaction norms, maternal effects
1. Introduction
Elaborate behavioural interactions between caring parents and their dependent offspring are common in animals where parents repeatedly provide their offspring with resources after hatching or birth (Clutton-Brock 1991). For example, parents of many birds and mammals, as well as some insects, adjust their provisioning of resources in response to conspicuous offspring begging displays. In these same systems, the offspring also adjust their begging behaviour in response to the amount of resources received from the parents (Kilner & Johnstone 1997; Budden & Wright 2001; Wright & Leonard 2002). The evolution of such complex and dynamic behavioural interactions between parents and offspring has been, and continues to be, a major area of research interest in behavioural ecology and quantitative genetics (Royle et al. 2002; Wright & Leonard 2002; Kölliker et al. 2005).
Traditionally, behavioural ecologists have considered the complex nature of behavioural interactions between parents and offspring as a phenotypic manifestation of parent–offspring conflict. Important issues in this field have been to understand how parental and offspring behaviours evolve given that parents and offspring have conflicting interests, and to understand how parental and offspring behaviours contribute to the resolution of conflict (Godfray 1995a; Kilner & Johnstone 1997; Wright & Leonard 2002; Royle et al. 2004). Meanwhile, quantitative geneticists have focused upon co-adaptation between parents and offspring resulting from the combined effects of parental and offspring behaviours on offspring fitness (Wolf & Brodie 1998; Kölliker et al. 2005; Wolf & Hager 2006). Previous reviews have compared the general value of the optimality approach used by behavioural ecologists and quantitative genetics (Moore & Boake 1994; Gomulkiewicz 1998). In our review, we compare the two approaches in the context of parent–offspring conflict and co-adaptation, and address the hitherto unresolved issue of how we might reconcile these two approaches to establish a common framework that combines central ideas and concepts from both sides.
Here, we first review the merits and limitations of traditional behavioural ecology and quantitative genetic approaches, and show that these two approaches provide valuable complementary insights into the evolution of parent–offspring interactions. We then outline a joint framework, based on the concept of behavioural reaction norms, and propose that this framework can be used to gain novel insights into the evolution of parent–offspring conflict and co-adaptation by incorporating information or assumptions about the genetic basis of parental and offspring behaviours while maintaining key insights into the functional and dynamic nature of parent–offspring interactions from behavioural ecology. While we present this synthesis with a specific focus on the evolution of parent–offspring interactions, we conclude that the proposed framework is valid and useful also in a broader context as a potential approach for advancing our understanding of the evolution of social interactions in general.
2. Behavioural ecology versus quantitative genetics
(a) Parent–offspring conflict and its resolution
Behavioural ecologists investigate parent–offspring interactions to gain insights into the evolutionary resolution of parent–offspring conflict. Parent–offspring conflict has been a key theoretical concept in behavioural ecology ever since Trivers (1974) first proposed the idea. Prior to Trivers, the presumption among evolutionary biologists was that parents and offspring had identical evolutionary interests regarding resource allocation. However, Trivers argued that, due to asymmetries in relatedness between parents and offspring, each offspring should be under selection to demand more than its fair share of the resources, because it is more closely related to itself than to its siblings. Conversely, parents should be under selection to attempt to divide resources equitably between their own offspring, because parents are equally related to all of them. Since Trivers, behavioural ecologists have explored the consequences of parent–offspring conflict for the evolution of parent–offspring interactions using a combination of game-theoretic modelling and behavioural experimentation.
Game-theoretic models based upon explicit assumptions about the behavioural strategies played by each family member have established that the adaptive function of elaborate parent–offspring interactions can be understood in the context of the resolution of parent–offspring conflict (Godfray 1995a). These models aim to identify evolutionarily stable pairs of parental resource provisioning and offspring begging strategies, and as such fall into two major classes: (i) honest signalling models, which assume that parents control the allocation of resources (Godfray 1991, 1995b) and (ii) scramble competition models, which assume that offspring control the allocation of resources (Parker & Macnair 1979; Parker et al. 2002a). Despite making opposite assumptions as to which party controls resource allocation, both classes of model share the prediction that begging ultimately reflects individual offspring need, thus providing parents with information on their offspring's nutritional needs. Distinguishing between honest signalling and scramble competition remains one of the major unresolved problems faced by behavioural ecologists studying parent–offspring interactions (Royle et al. 2002).
Behavioural ecologists have conducted a wealth of experimental studies over the past decades, thereby providing important insights into the adaptive function of dynamic parent–offspring interactions. Food-deprivation experiments designed to test whether offspring adjust their begging behaviour to changes in their hunger levels have now been conducted across a wide range of taxa. Such experiments show the expected general pattern that offspring respond by begging more when they are food deprived and hungry than when they are satiated (Kilner & Johnstone 1997; Budden & Wright 2001; Wright & Leonard 2002; Smiseth & Moore 2004). However, other experiments suggest that offspring begging is not simply a signal of the offspring's hunger state, but is influenced by a number of additional factors, including the long-term need for food (Price et al. 1996), parasite infestation (Christe et al. 1996), immunocompetence (Saino & Møller 2002), the number of offspring in the brood (e.g. Leonard et al. 2000), competitive rank (e.g. Cotton et al. 1999) and learning experiences (Kedar et al. 2000). With regard to parental responses, experiments have also shown a general pattern that parents adjust their resource provisioning according to the changes in offspring begging levels (Kilner & Johnstone 1997; Budden & Wright 2001; Wright & Leonard 2002). However, parents also adjust their provisioning behaviour to additional factors. For instance, in species with bi-parental care, parents often adjust their food provisioning to the amount of care provided by their partner, suggesting that parents somehow negotiate the amount of care that each should provide (Wright & Cuthill 1989; Houston et al. 2005).
The strength of the optimality approach taken by behavioural ecologists is that it is explicit about the consequences of parent–offspring conflict and the adaptive function of parent–offspring communication as a behavioural mechanism for conflict resolution. The limitation of this approach is that it is based on the assumptions that the evolution of parental and offspring behaviours can be understood from a purely phenotypic perspective, and that information about genetic architecture, including genetic correlations between traits, can be safely ignored (Grafen 1991). This approach, which was termed ‘the phenotypic gambit’ by Grafen (1991), has allowed behavioural ecologists to greatly expand our understanding of the evolutionary function of animal behaviour over the past four decades without the need for detailed and laborious studies on trait inheritance and genetic architecture. However, the behavioural ecology approach ultimately rests on the implicit assumptions that (i) traits have a heritable basis (otherwise they would not evolve), (ii) parental and offspring behaviours have an independent genetic basis and (iii) parental and offspring behaviours as observed today have reached an evolutionarily stable equilibrium. There is increasing experimental evidence for maintained heritable variation in, and genetic correlations between, parental and offspring behaviours, which suggests that one or more of these assumptions may be violated (reviewed in Kölliker & Richner 2001 and Kölliker 2005). Thus, in order to advance our understanding of the evolution of parent–offspring interactions, we now need to explicitly incorporate information on the genetic basis of parental and offspring behaviours. Quantitative genetics provides the necessary theoretical concepts and tools for obtaining such information.
(b) Maternal effects, offspring effects and co-adaptation
Quantitative genetics aims to predict evolutionary responses to selection on a given trait based upon information or assumptions about the form and strength of selection and patterns of trait inheritance (Lande & Arnold 1983; Lynch & Walsh 1998). Given that selection can act on multiple traits simultaneously, the evolutionary response to selection for a given trait depends not only on the genetic variation in the trait in question, but also on the genetic covariances with other traits (Lande & Arnold 1983; Lynch & Walsh 1998). Selection is usually estimated in terms of selection gradients obtained as partial regression coefficients in multiple regression models, and can be further differentiated into linear (i.e. directional) or nonlinear (e.g. stabilizing) selection gradients (Lande & Arnold 1983). Information or assumptions about genetic architecture, such as the genetic variances and covariances of traits, are often assumed to be constant when predicting the evolution of mean trait values. In addition, information or assumptions about patterns of selection on suites of traits can also be incorporated to explore evolutionary change in the genetic variances and covariances of multiple traits (Phillips & McGuigan 2006).
Quantitative genetics models examine the evolution of parental provisioning of dependent offspring in terms of adaptive maternal effects, defined as environmental conditions generated by the parent that influences the expression of offspring traits such as growth and survival (Cheverud 1984; Kirkpatrick & Lande 1989; Cheverud & Moore 1994; Mousseau & Fox 1998; Räsänen & Kruuk 2007). Although maternal effects are defined as environmental sources of variation, they are likely to have a partially genetic basis, in which case they might evolve in response to selection (Kirkpatrick & Lande 1989). Quantitative genetics models have generally ignored the possibility that offspring may actively influence parental contributions to their growth and survival, for example, through engaging in conspicuous begging displays (Kilner & Johnstone 1997; Wright & Leonard 2002). However, the recent models suggest that offspring begging can be incorporated into such models by treating it as an offspring effect that influences parental provisioning, thus generating a more realistic and complex feedback loop between parental and offspring behaviours (Kölliker 2003; Kölliker et al. 2005).
Quantitative genetics models suggest that patterns of genetic variation and covariation in parental and offspring traits can reveal important information about patterns of past selection (Wolf & Brodie 1998), such as the nature of particular evolutionary resolutions to parent–offspring conflict (Kölliker et al. 2005). Maternal and offspring effects can generate epistasis for fitness; i.e. particular combinations of parental and offspring traits with similar fitness values (Wolf & Brodie 1998; Wolf 2000; Kölliker et al. 2005). Such epistasis for fitness is expected to select for co-adaptation between parents and offspring, which can be detected as a genetic correlation between parental and offspring trait values. If parental provisioning is the trait under selection, the sign of such genetic correlations is usually expected to be negative, because parents are adapting to genetic variation in offspring begging behaviour (Kölliker et al. 2005). Conversely, if offspring begging is under selection, the sign of such correlations is expected to be positive, because now it is offspring that is adapting to genetic variation in parental provisioning (Kölliker et al. 2005).
Interestingly, the recent experimental work across a wide range of taxa has provided evidence for such variation in patterns of co-adaptation between parental provisioning of resources and offspring demand for resources (Kölliker et al. 2000; Agrawal et al. 2001; Hager & Johnstone 2003; Curley et al. 2004; Lock et al. 2004; Maestripieri 2004; Qvarnström et al. 2007). In particular, cross-fostering experiments have reported a negative genetic correlation between parental provisioning and brood demand in burrower bugs (Sehirus cinctus; Agrawal et al. 2001) and rhesus macaques (Macaca mulatta; Maestripieri 2004), and a positive genetic correlation between parental provisioning and offspring begging in great tits (Panus major; Kölliker et al. 2000) and burying beetles (Nicrophorus vespilloides; Lock et al. 2004). Further evidence for inherited patterns in parent–offspring interactions comes from an interspecific partial cross-fostering experiment on pied and collared flycatchers, where the divergence in begging between the two species reflected a divergence in offspring need for resources (Qvarnström et al. 2007). Such studies therefore suggest that quantitative genetics models provide valuable insight into patterns of co-adaptation between parental and offspring trait values.
The strength of the approach taken by quantitative geneticists is that it allows us to explicitly incorporate information or assumptions about the heritable basis of parental and offspring behaviours to predict evolutionary changes in these traits. Furthermore, the approach allows us to examine the evolution of genetic variances and covariances, thereby providing valuable insights into the evolutionary consequences of co-adaptation between parents and offspring. Both the heritable basis of parental and offspring behaviours and the co-adaptation between parents and offspring have hitherto been largely ignored by behavioural ecologists. Finally, quantitative genetics is explicit about the definition and description of parental and offspring fitness as components of an individual's lifetime fitness, while behavioural ecology can be ambiguous as to whether particular fitness components, such as offspring growth and survival, should be assigned to parents or offspring (Cheverud & Moore 1994; Wolf & Wade 2001). A major limitation to the quantitative genetics approach, however, is that it ignores the influences of antagonistic selection caused by parent–offspring conflict, and thus the functional context in which parental and offspring behaviours evolve (i.e. conflict resolution). Instead, parent–offspring interactions are defined rather broadly as maternal and offspring effects, and the links with the adaptive function of parent–offspring communication as a behavioural mechanism for conflict resolution are unclear. Furthermore, quantitative genetics is based upon very explicit and limited definitions of parental and offspring traits. It thereby ignores the complex and often dynamic nature of parental provisioning and offspring begging behaviours, including changes due to social environment, ecological conditions, development, age or learning (Kilner & Johnstone 1997; Wright & Leonard 2002), unless the models are specifically modified to handle such effects. For example, Lock et al. (2007) found that pre- and postnatal maternal effects changed in opposite directions as a function of female age in the burying beetle N. vespilloides, thus illustrating that maternal care should be treated as a complex suite of co-adapted traits.
3. A combined approach: behavioural reaction norms
It is clear that behavioural ecology and quantitative genetics provide important complementary insights concerning the evolution of parent–offspring interactions, and that in the future both approaches will undoubtedly develop further due to continued interest in the evolution of parent–offspring conflict and co-adaptation. However, we feel that to further advance our understanding of the evolution of parent–offspring interactions, it is important to integrate these two traditional approaches, and that this is best done using a behavioural reaction norm approach (see also Taylor & Day 2004), which combines theoretical concepts and empirical approaches of behavioural ecology and quantitative genetics.
Reaction norms are used in quantitative genetics to account for the impact of environmental effects on the phenotype produced by a given genotype (e.g. Kirkpatrick & Heckman 1989; de Jong 1990; Gomulkiewicz & Kirkpatrick 1992) and their evolution can be modelled as evolving function-valued traits (Kingsolver et al. 2001; Kirkpatrick & Meyer 2004; Meyer & Kirkpatrick 2005). Reaction norms have rarely been used to conceptualize the evolution of social behaviours (Agrawal 2001), although the expression of many social behaviours would fit the definition of a reaction norm or a function-valued trait. Behavioural reaction norms are similar to traditional reaction norms, but are different in one important respect. Traditional reaction norms involve irreversible or slowly developing effects of the physical environment on the phenotype (i.e. phenotypic plasticity; Piersma & Drent 2003), such as the effect of temperature on growth and other developmental traits (Nussey et al. 2007). By contrast, behavioural reaction norms describe fast responses by the focal individual to variation in the social environment (Agrawal 2001). This involves repeated interactions with reversible effects and temporal behavioural dynamics, such as the time elapsed since the offspring's last feeding event (i.e. phenotypic flexibility; Piersma & Drent 2003). In addition, the concept of behavioural reaction norms is very similar to that of response functions or negotiation rules, which are commonly used in behavioural ecology (Taylor & Day 2004; Houston et al. 2005). The two concepts differ in the sense that only the former term explicitly incorporates information on the heritable basis of behaviour.
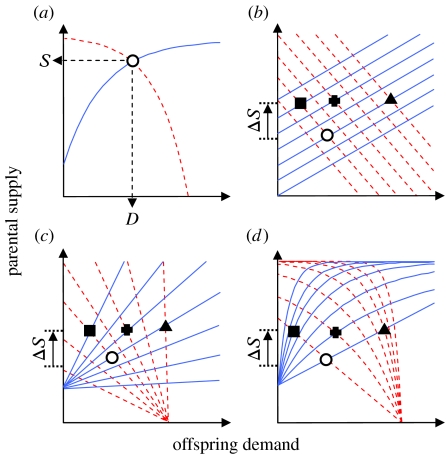
Behavioural reaction norms map very easily onto traditional perspectives of parent–offspring interactions. It was Hussell (1988) who first proposed a model for the proximate basis of parent–offspring interactions based upon behavioural response functions (figure 1a), which define the rules by which parents and offspring react to each other's behaviours in terms of supply and demand. The offspring response function describes the effect that provisioning of resources by parents has on offspring begging behaviour (i.e. the demand function, or the effect of supply on demand). The parent response function describes the effect that offspring begging behaviour has on the provisioning effort of the parents (i.e. supply function, or the effect of demand on supply). Hussell's (1988) model has subsequently been used as the conceptual basis for the idea that parent–offspring interactions provide a behavioural mechanism for the resolution of parent–offspring conflict, whereby parents and offspring dynamically react to each other's behaviours. As such, Hussell's (1988) supply and demand functions are equivalent to the behavioural reaction norms when expressed as behavioural responses with heritable variation in the intercepts, slopes or curvilinear shapes (figure 1). This connection between response functions and behavioural reaction norms helps bridge the empirical, mathematical and terminological gap between behavioural ecology and quantitative genetics (Johnstone 1996; Mock & Parker 1997; Parker et al. 2002b; Kölliker 2003).
Figure 1.
Illustration of parental supply (solid blue lines) and offspring demand (dashed red lines) functions, and the evolution of parental provisioning and offspring begging as interacting behavioural reaction norms. (a) A simple example in which the observed supply (S) and demand (D) levels are defined at the intersect. Genetic variation between individuals can occur as variation in (b) intercept, (c) slope and (d) shape of the functions. Each intersect represents a possible outcome of the supply–demand interaction. Assuming an ancestral population with mean values represented by the circle, an increase in the level of parental provisioning by ΔS can be produced via changes in the supply function's intercept (b; square—increased parental supply to all levels of offspring begging), slope (c; square—higher parental responsiveness to begging) or shape (d; square—higher responsiveness to begging especially at low levels). In these cases, the behavioural level of offspring begging is assumed to passively follow according to an evolutionarily unchanged demand function. Alternatively, an increase in the level of parental provisioning by ΔS could also be achieved through evolutionary changes in the demand function intercept (b; triangle—increased offspring begging to all levels of parental supply), slope (c; triangle—reduced offspring sensitivity to supply) or shape (d; triangle—reduced sensitivity to supply especially at low levels). In the latter three scenarios, parental provisioning is assumed to passively follow according to an evolutionarily unchanged supply function. Finally, an increase in the level of parental provisioning by ΔS could occur due to changes in either intercept (b; plus), slope (c; plus) or shape (d; plus) of both parental and offspring response functions.
The recent theoretical developments in behavioural ecology highlight the key conceptual role of Hussell's (1988) proximate model in the modelling of parent–offspring conflict (Johnstone 1996; Mock & Parker 1997; Parker et al. 2002b). Different models for the resolution of parent–offspring conflict are often based on different assumptions about the offspring demand function (Mock & Parker 1997; Parker et al. 2002b). For example, scramble competition models assume that the offspring demand function is negative; that is, offspring beg less when parents provide more food (Parker & Macnair 1979; Parker et al. 2002b). Conversely, honest signalling models assume that the offspring demand function is zero; that is, parental food provisioning has no effect on offspring begging (Godfray 1991, 1995b). A more recent version of the honest signalling model assumes that the offspring demand function is negative (Johnstone 1996). Interestingly, these theoretical developments show that assumptions about the shape of the offspring demand function affect the outcome of parent–offspring conflict because, in honest signalling models, the allocation of resources is shifted away from the parents' optimum and towards the offspring's optimum when the offspring demand function is assumed to be negative as compared with when it is assumed to be zero (Johnstone 1996).
The relevance of Hussell's (1988) proximate supply demand model to a quantitative genetics theory of parent–offspring co-adaptation becomes apparent when this purely graphical model is formalized using linear regression equations (Kölliker 2003). By expressing supply and demand functions in terms of additive genetic values (i.e. breeding values), their evolution can be modelled from a quantitative genetics perspective (figure 1). Co-adaptation models of this sort assume that the intercepts of the linear supply and demand functions are evolving, but that their shapes (i.e. slopes) are fixed and are not subject to evolutionary change (Wolf & Brodie 1998; Kölliker et al. 2005). Such models therefore do not treat these functions as fully coevolving behavioural reactions norms. This is because, just as the shapes of the supply and demand functions are crucial in conflict resolution models, the predicted pattern of co-adaptation depends critically not only on the patterns of selection but also on the assumed slopes of the response functions (Wolf & Brodie 1998; Kölliker et al. 2005).
The behavioural reaction norm approach we are advocating differs from existing conflict resolution and co-adaptation approaches by focusing explicitly on the coevolution of the behavioural response rules (McNamara et al. 1999; Taylor & Day 2004; Houston et al. 2005). It thereby places selection on the temporal and behavioural dynamics of such interactions at the very centre of any investigation of parent–offspring conflict. Our current understanding of parent–offspring interactions is almost entirely based upon simplistic and inappropriate static arguments, models and evidence (e.g. Godfray & Johnstone 2000). Therefore, any future research (both theoretical and experimental) that uses a behavioural reaction norm approach will automatically provide a solution to this widely recognized problem, while also combining the best of traditional behavioural ecology and quantitative genetics approaches.
4. Applications of the behavioural reaction norm approach
Now we have three complementary approaches to the study of parent–offspring interactions: the traditional behavioural ecology and quantitative genetics approaches, and the combined behavioural reaction norm approach presented here. All three approaches should be pursued in the future, because the suitability of the approach taken will depend upon the type of question under scrutiny. For example, traditional behavioural ecology is likely to be best for studying the mechanisms of conflict resolution, while traditional quantitative genetics will continue to provide insights into co-adaptation between parents and offspring. The behavioural reaction norm approach will be of most use in tackling crucial questions regarding the evolution of parent–offspring interactions, which is a key area that has been left unexplored by the traditional approaches.
The behavioural reaction norm framework makes clear that the evolving traits in parent–offspring interactions are how parents and offspring respond to each other. Direct support for this central evolutionary role of offspring and parent behavioural reaction norms comes from the traditional resolution and co-adaptation models themselves: in both types of models, assumed variation in the slopes of the demand and/or supply response function generates variation in offspring and/or parental fitness (Wolf & Brodie 1998; Parker et al. 2002b; Kölliker et al. 2005). Thus, previous models have demonstrated selection on parental and offspring behavioural reaction norms, but there was no evolutionary effect of selection because the response rules were assumed to be fixed. Only by turning fixed response rules into heritable and evolving behavioural reaction norms can selection be allowed to have the required evolutionary consequences. Appropriate evolutionary models are now needed to formalize a theory of coevolving behavioural reaction norms in the context of parent–offspring interactions. Such models may be founded around and extended upon negotiation models (McNamara et al. 1999; Taylor & Day 2004), or upon reaction norms (Kirkpatrick & Heckman 1989; de Jong 1990; Gomulkiewicz & Kirkpatrick 1992) and other types of function-valued trait models (Kingsolver et al. 2001; Kirkpatrick & Meyer 2004; Meyer & Kirkpatrick 2005).
In experimental research, perhaps the most obvious advantage of the behavioural reaction norm approach is that it provides a sound and intuitive foundation to study the heritable basis of parental resource provisioning and offspring begging. In contrast to traditional quantitative genetics, which would estimate genetic variation in the level of parental resource provisioning and offspring begging, the behavioural reaction norm approach can be used to estimate genetic variation in the nature of parental and offspring response functions. Such information would be essential given that the expression of these behaviours is dynamic and depends critically on the behaviour of the other party (Kilner & Johnstone 1997; Budden & Wright 2001; Wright & Leonard 2002). The first step in this process would involve experimental assessment of the intercept, slope and shape of both parental and offspring reaction norms by measuring the begging of individual offspring under various levels of food deprivation, and the provisioning of individual parents under various levels of offspring begging (Kilner & Johnstone 1997; Budden & Wright 2001; Wright & Leonard 2002; Kölliker 2003). The next step would be to conduct such experimental tests within traditional quantitative genetics breeding designs to obtain estimates of the heritable basis of parental and offspring behavioural reaction norms. Such studies would provide new and important information concerning the amount of genetic variation in the different components of parental supply and offspring demand reaction norms, i.e. the intercept, slope and shape, as well as the level of genetic correlation between these components.
The behavioural reaction norm approach would provide important new information concerning any selection on and evolution of parental supply and offspring demand response functions. Traditional quantitative genetics and behavioural ecology approaches implicitly assume that parental and offspring response functions are fixed over evolutionary time (Godfray 1991, 1995a,b; Wolf & Brodie 1998; Parker et al. 2002a,b; Kölliker et al. 2005). Yet, parents and offspring are also assumed to change their behaviour adaptively in response to variation in each other's behaviour, thus implying that response functions have an adaptive function. It is therefore important to conduct experiments investigating how selection and inheritance can affect the evolution of parental supply and offspring demand reaction norms (figure 1). Given the complex and dynamic nature of the behaviours involved, such experiments represent a major empirical challenge, necessitating the establishment of new experimental systems alongside the well-established bird and mammal model systems. This would have to include laboratory systems with short generation times that allow us to conduct artificial breeding, artificial selection and experimental evolution experiments. Suitable insect species, such as the burying beetle N. vespilloides (Smiseth & Moore 2004) and the earwig Forficula auricularia (Kölliker 2007), perhaps represent the most promising systems to this end. In addition, it should be possible to assess the historical effects of selection via comparative studies, an approach only very rarely exploited in the study of parent–offspring interactions. Earlier studies on nestling begging in birds by Briskie et al. (1994) and Haskell (1999) have shown that loudness begging of calls is negatively correlated with variation in within-brood relatedness (due to extra-pair mating) and the probability of nest predation (due to nest vulnerability), as predicted by parent–offspring conflict theory. More specific theoretical predictions could be generated concerning the exact shapes of behavioural reaction norms concerning parental provisioning responses and offspring begging sensitivity, and tested for via food deprivation and/or begging playback experiments in species with contrasting mating systems, life history and ecology.
The behavioural reaction norm approach we advocate for the study of parent–offspring interactions could also be applied to other questions where the expression of traits depends upon the behaviour of other individuals. Behavioural ecologists have demonstrated that social behaviours in general tend to be flexible in the sense that focal individuals adjust their behaviour to the behaviour of other individual(s) involved in the interaction (Moore et al. 1997). Such behavioural flexibility encompasses the whole field of animal communication (Bradbury & Vehrencamp 1998), as well as maternal hormonal effects on offspring behavioural development (e.g. Müller et al. 2007), the evolution of cooperation (Taylor & Day 2004), aggressive behaviours expressed during escalated contests when competing for mates and resources (Huntingford & Turner 1987), courtship displays and mate choice (Andersson 1994; Chenoweth & Blows 2006), plus cooperative anti-predator vigilance and mobbing (Caro 2005) and cooperative breeding (Koenig & Dickenson 2004). We suggest that the behavioural reaction norm approach could be applied to all of these situations, possibly providing a generally valid common framework for behavioural ecologists and quantitative geneticists to study the genetic evolution of social interactions. While we suggest that parent–offspring interactions provide the most obvious starting point for the development of such an approach, there is clearly potential to expand this way of thinking more generally when studying the evolution of a range of different social behaviours.
Acknowledgments
We thank Ralph Dobler, Roi Dor, Uri Grodzinski, Andy Horn, Becky Kilner, Marty Leonard, Arnon Lotem, Flore Mas, Katja Räsänen, Jason Wolf and two anonymous referees for their valuable comments on the manuscript. Financial support for the study was provided from NERC as a fellowship to P.T.S., and from the Swiss NSF as a research grant to M.K.
References
- Agrawal A.A. Phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. doi:10.1126/science.1060701 [DOI] [PubMed] [Google Scholar]
- Agrawal A.F, Brodie E.D, III, Brown J. Parent–offspring coadaptation and the duel genetic control of maternal care. Science. 2001;292:1710–1712. doi: 10.1126/science.1059910. doi:10.1126/science.1059910 [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bradbury J.W, Vehrencamp S.L. Sinauer Associates, Inc; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Briskie J.V, Naugler C.T, Leech S.M. Begging intensity of nestling birds varies with sibling relatedness. Proc. R. Soc. B. 1994;258:73–78. doi:10.1098/rspb.1994.0144 [Google Scholar]
- Budden A.E, Wright J. Begging in nestling birds. Curr. Ornithol. 2001;16:83–118. [Google Scholar]
- Caro T. The University of Chicago Press; Chicago, IL: 2005. Antipredator defenses in birds and mammals. [Google Scholar]
- Chenoweth S.F, Blows M.W. Dissecting the complex genetic basis of mate choice. Nat. Rev. Genet. 2006;7:681–692. doi: 10.1038/nrg1924. doi:10.1038/nrg1924 [DOI] [PubMed] [Google Scholar]
- Cheverud J.M. Evolution by kin selection: a quantitative genetic model illustrated by maternal performance in mice. Evolution. 1984;38:766–777. doi: 10.1111/j.1558-5646.1984.tb00349.x. doi:10.2307/2408388 [DOI] [PubMed] [Google Scholar]
- Cheverud J.M, Moore A.J. Quantitative genetics and the role of the environment provided by relatives in behavioural evolution. In: Boake C.R.B, editor. Quantitative genetics studies of behavioral evolution. University of Chicago Press; Chicago, IL: 1994. pp. 67–100. [Google Scholar]
- Christe P, Richner H, Oppliger A. Begging, food provisioning and nestling competition in great tit broods infested with ectoparasites. Behav. Ecol. 1996;7:127–131. doi:10.1093/beheco/7.2.127 [Google Scholar]
- Cotton P.A, Wright J, Kacelnik A. Chick begging strategies in relation to brood hierarchies and hatching asynchrony. Am. Nat. 1999;153:412–420. doi: 10.1086/303178. doi:10.1086/303178 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Curley J.P, Barton S, Surani A, Keverne E.B. Coadaptation in mother and infant regulated by a paternally expressed imprinted gene. Proc. R. Soc. B. 2004;271:1303–1309. doi: 10.1098/rspb.2004.2725. doi:10.1098/rspb.2004.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong G. Quantitative genetics of reaction norms. J. Evol. Biol. 1990;3:447–468. doi:10.1046/j.1420-9101.1990.3050447.x [Google Scholar]
- Godfray H.C.J. Signalling of need by offspring to their parents. Nature. 1991;352:328–330. doi:10.1038/352328a0 [Google Scholar]
- Godfray H.C.J. Evolutionary theory of parent–offspring conflict. Nature. 1995a;376:133–138. doi: 10.1038/376133a0. doi:10.1038/376133a0 [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 1995b;146:1–24. doi:10.1086/285784 [Google Scholar]
- Godfray H.C.J, Johnstone R.A. Begging and bleating: the evolution of parent–offspring signalling. Phil. Trans. R. Soc. B. 2000;355:1581–1591. doi: 10.1098/rstb.2000.0719. doi:10.1098/rstb.2000.0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomulkiewicz R. Game theory, optimization, and quantitative genetics. In: Dugatkin L.A, Reeve H.K, editors. Game theory and animal behaviour. Oxford University Press; Oxford, UK: 1998. pp. 283–303. [Google Scholar]
- Gomulkiewicz R, Kirkpatrick M. Quantitative genetics and evolution of reaction norms. Evolution. 1992;46:390–411. doi: 10.1111/j.1558-5646.1992.tb02047.x. doi:10.2307/2409860 [DOI] [PubMed] [Google Scholar]
- Grafen A. Modelling in behavioural ecology. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell Science; Oxford, UK: 1991. pp. 5–31. [Google Scholar]
- Hager R, Johnstone R.A. The genetic basis of family conflict resolution in mice. Nature. 2003;421:533–535. doi: 10.1038/nature01239. doi:10.1038/nature01239 [DOI] [PubMed] [Google Scholar]
- Haskell D.G. The effect of predation on begging-call evolution in nestling wood warblers. Anim. Behav. 1999;57:893–901. doi: 10.1006/anbe.1998.1053. doi:10.1006/anbe.1998.1053 [DOI] [PubMed] [Google Scholar]
- Houston A.I, Székely T, McNamara J.M. Conflict between parents over care. Trends Ecol. Evol. 2005;20:33–38. doi: 10.1016/j.tree.2004.10.008. doi:10.1016/j.tree.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Huntingford F, Turner A. Chapman and Hall; London, UK: 1987. Animal conflict. [Google Scholar]
- Hussell D.J.T. Supply and demand in tree swallow broods: a model of parent–offspring food-provisioning interactions in birds. Am. Nat. 1988;131:175–202. doi:10.1086/284785 [Google Scholar]
- Johnstone R.A. Begging signals and parent–offspring conflict: do parents always win? Proc. R. Soc. B. 1996;263:1677–1681. doi:10.1098/rspb.1996.0245 [Google Scholar]
- Kedar H, Rodriguez-Girone`s M.A, Yedvab S, Lotem A, Winkler D.W. Experimental evidence for offspring learning in parent–offspring communication. Proc. R. Soc. B. 2000;267:1723–1727. doi: 10.1098/rspb.2000.1201. doi:10.1098/rspb.2000.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R, Johnstone R.A. Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol. Evol. 1997;12:11–15. doi: 10.1016/s0169-5347(96)10061-6. doi:10.1016/S0169-5347(96)10061-6 [DOI] [PubMed] [Google Scholar]
- Kingsolver J.G, Gomulkiewicz R, Carter P.A. Variation, selection and evolution of function-valued traits. Genetica. 2001;112-113:87–104. doi:10.1023/A:1013323318612 [PubMed] [Google Scholar]
- Kirkpatrick M, Heckman N. A quantitative genetics model for growth, shape, reaction norms, and other infinite-dimensional characters. J. Math. Biol. 1989;27:429–450. doi: 10.1007/BF00290638. doi:10.1007/BF00290638 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;43:485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. doi:10.2307/2409054 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Meyer K. Direct estimation of genetic principle components: simplified analysis of complex phenotypes. Genetics. 2004;168:2295–2306. doi: 10.1534/genetics.104.029181. doi:10.1534/genetics.104.029181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W.D, Dickenson J.L. Cambridge University Press; Cambridge, UK: 2004. Ecology and evolution of cooperative breeding in birds. [Google Scholar]
- Kölliker M. Estimating mechanisms and equilibria for offspring begging and parental provisioning. Proc. R. Soc. B. 2003;270:S110–S113. doi: 10.1098/rsbl.2003.0032. doi:10.1098/rsbl.2003.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker M. Ontogeny in the family. Behav. Genet. 2005;35:7–18. doi: 10.1007/s10519-004-0852-9. doi:10.1007/s10519-004-0852-9 [DOI] [PubMed] [Google Scholar]
- Kölliker M. Benefits and costs of earwig (Forficula auricularia) family life. Behav. Ecol. Sociobiol. 2007;61:1489–1497. doi:10.1007/s00265-007-0381-7 [Google Scholar]
- Kölliker M, Richner H. Parent–offspring conflict and the genetics of offspring solicitation and parental response. Anim. Behav. 2001;62:395–407. doi:10.1006/anbe.2001.1792 [Google Scholar]
- Kölliker M, Brinkhof M.W.G, Heeb P, Fitze P.S, Richner H. The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc. R. Soc. B. 2000;267:2127–2132. doi: 10.1098/rspb.2000.1259. doi:10.1098/rspb.2000.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker M, Brodie E.D, III, Moore A.J. The coadaptation of parental supply and offspring demand. Am. Nat. 2005;166:506–516. doi: 10.1086/491687. doi:10.1086/491687 [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold S.J. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. doi:10.2307/2408842 [DOI] [PubMed] [Google Scholar]
- Leonard M, Horn A.G, Gozna A, Ramen S. Brood size and begging intensity in nestling birds. Behav. Ecol. 2000;11:196–201. doi:10.1093/beheco/11.2.196 [Google Scholar]
- Lock J.E, Smiseth P.T, Moore A.J. Selection, inheritance, and the evolution of parent–offspring interactions. Am. Nat. 2004;164:13–24. doi: 10.1086/421444. doi:10.1086/421444 [DOI] [PubMed] [Google Scholar]
- Lock J.E, Smiseth P.T, Moore P.J, Moore A.J. Coadaptation of prenatal and postnatal maternal effects. Am. Nat. 2007;170:709–718. doi: 10.1086/521963. doi:10.1086/521963 [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Sinauer Associates, Inc; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- Maestripieri D. Genetic aspects of mother–offspring conflict in rhesus macaques. Behav. Ecol. Sociobiol. 2004;55:381–387. doi:10.1007/s00265-003-0720-2 [Google Scholar]
- McNamara J.M, Gasson C.E, Houston A.I. Incorporating rules for responding into evolutionary games. Nature. 1999;401:368–371. doi: 10.1038/43869. [DOI] [PubMed] [Google Scholar]
- Meyer K, Kirkpatrick M. Up hill, down dale: quantitative genetics of curvaceous traits. Phil. Trans. R. Soc. B. 2005;360:1443–1455. doi: 10.1098/rstb.2005.1681. doi:10.1098/rstb.2005.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock D.W, Parker G.A. Oxford University Press; Oxford, UK: 1997. The evolution of sibling rivalry. [Google Scholar]
- Moore A.J, Boake C.R.B. Optimality and evolutionary genetics: complementary procedures for evolutionary analysis in behavioural ecology. Trends Ecol. Evol. 1994;9:69–72. doi: 10.1016/0169-5347(94)90278-X. doi:10.1016/0169-5347(94)90278-X [DOI] [PubMed] [Google Scholar]
- Moore A.J, Brodie E.D, III, Wolf J.B. Interacting phenotypes and the evolutionary process: I. Direct and indirect genetic effects of social interactions. Evolution. 1997;51:1352–1362. doi: 10.1111/j.1558-5646.1997.tb01458.x. doi:10.2307/2411187 [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C. Oxford University Press; Oxford, UK: 1998. Maternal effects as adaptations. [Google Scholar]
- Müller W, Lessells C.M, Korsten P, von Engelhardt N. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am. Nat. 2007;169:E84–E96. doi: 10.1086/511962. doi:10.1086/511962 [DOI] [PubMed] [Google Scholar]
- Nussey D.H, Wilson A.J, Brommer J.E. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 2007;20:831–844. doi: 10.1111/j.1420-9101.2007.01300.x. doi:10.1111/j.1420-9101.2007.01300.x [DOI] [PubMed] [Google Scholar]
- Parker G.A, Macnair M.R. Models of parent–offspring conflict. IV. Suppression: evolutionary retaliation by the parent. Anim. Behav. 1979;27:1210–1235. doi:10.1016/0003-3472(79)90068-X [Google Scholar]
- Parker G.A, Royle N.J, Hartley I.R. Begging scrambles with unequal chicks: interactions between need and competitive ability. Ecol. Lett. 2002a;5:206–215. doi:10.1046/j.1461-0248.2002.00301.x [Google Scholar]
- Parker G.A, Royle N.J, Hartley I.R. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. B. 2002b;357:295–307. doi: 10.1098/rstb.2001.0950. doi:10.1098/rstb.2001.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P.C, McGuigan K.L. Evolution of genetic variance–covariance structure. In: Fox C.W, Wolf J.B, editors. Evolutionary genetics. Oxford University Press; Oxford, UK: 2006. pp. 310–325. [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003;18:228–233. doi:10.1016/S0169-5347(03)00036-3 [Google Scholar]
- Price K, Harvey H, Ydenberg R. Begging tactics of yellow-headed blackbirds, Xanthocephalus xanthocephalus, in relation to need. Anim. Behav. 1996;51:421–435. doi:10.1006/anbe.1996.0039 [Google Scholar]
- Qvarnström A, Vogel Kehlenbeck J, Wiley C, Svedin N, Sæther S.A. Species divergence in offspring begging intensity: difference in need of manipulation of parents? Proc. R. Soc. B. 2007;274:1003–1008. doi: 10.1098/rspb.2006.0255. doi:10.1098/rspb.2006.0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räsänen K, Kruuk L.E.B. Maternal effects and evolution at ecological time scales. Funct. Ecol. 2007;21:408–421. doi:10.1111/j.1365-2435.2007.01246.x [Google Scholar]
- Royle N.J, Hartley I.R, Parker G.A. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 2002;17:434–440. doi:10.1016/S0169-5347(02)02565-X [Google Scholar]
- Royle N.J, Hartley I.R, Parker G.A. Parental investment and family dynamics: interactions between theory and empirical tests. Popul. Ecol. 2004;46:231–241. doi:10.1007/s10144-004-0196-6 [Google Scholar]
- Saino N, Møller A.P. Immunity and begging. In: Wright J, Leonard M, editors. The evolution of begging: competition, cooperation and communication. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. pp. 245–267. [Google Scholar]
- Smiseth P.T, Moore A.J. Signalling of hunger when offspring forage by both begging and self-feeding. Anim. Behav. 2004;67:1083–1088. doi:10.1016/j.anbehav.2003.10.012 [Google Scholar]
- Taylor P.D, Day T. Stability in negotiation games and the emergence of cooperation. Proc. R. Soc. B. 2004;271:669–674. doi: 10.1098/rspb.2003.2636. doi:10.1098/rspb.2003.2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L. Parent–offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- Wolf J.B. Gene interactions from maternal effects. Evolution. 2000;54:1882–1898. doi: 10.1111/j.0014-3820.2000.tb01235.x. doi:10.1554/0014-3820(2000)054[1882:GIFME]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wolf J.B, Brodie E.D., III The coadaptation of parental and offspring characters. Evolution. 1998;52:299–308. doi: 10.1111/j.1558-5646.1998.tb01632.x. doi:10.2307/2411068 [DOI] [PubMed] [Google Scholar]
- Wolf J.B, Hager R. A maternal–offspring coadaptation theory for the evolution of genomic imprinting. PLoS Biol. 2006;4:2238–2243. doi: 10.1371/journal.pbio.0040380. doi:10.1371/journal.pbio.0040380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J.B, Wade M.J. On the assignment of fitness to parents and offspring: whose fitness is it and does it matter? J. Evol. Biol. 2001;14:347–356. doi:10.1046/j.1420-9101.2001.00277.x [Google Scholar]
- Wright J, Cuthill I. Manipulation of sex differences in parental care. Behav. Ecol. Sociobiol. 1989;25:171–181. doi:10.1007/BF00302916 [Google Scholar]
- Wright J, Leonard M.L. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. The evolution of begging: competition, cooperation and communication. [Google Scholar]