Abstract
Most temperate-zone species use photoperiod to coordinate breeding and ensure that offspring are born during favourable conditions. Although photoperiodic influences on the reproductive axis have been well characterized, the precise mechanisms by which photoperiodic information and other seasonal cues are integrated to regulate reproductive function remain less well specified. Two recently discovered neuropeptides, kisspeptin and gonadotropin-inhibitory hormone, have pronounced opposing influences on reproductive function. This paper will review recent evidence for a role of these peptides in seasonal reproduction and propose a theoretical framework by which these novel regulatory peptides may serve to regulate seasonal breeding. Understanding the mechanisms regulating appropriate changes in reproductive status will serve to advance a wide range of life science disciplines.
Keywords: kisspeptin, gonadotropin-inhibitory hormone, seasonal reproduction, RFamide, RFamide-related peptide, metastin
1. Introduction
Understanding the mechanisms that regulate reproductive function has important implications for diverse fields from developmental biology, neuroscience and psychobiology to behavioural ecology and evolution. This review will focus on two recently identified neuropeptides, kisspeptin and gonadotropin-inhibitory hormone (GnIH). A brief overview of these peptides will be provided, followed by a hypothetical framework that describes how these hormones may function, both independently and in concert, to regulate vertebrate reproduction, with a specific emphasis placed on seasonal reproduction. The current review is primarily focused on the proximate level of analysis; however, the hope is that it will inform studies focused on reproduction from an ultimate, evolutionary perspective, by providing a mechanistic framework for appropriate timing of reproduction.
Most animal species experience marked variations in environmental conditions throughout the year. To maximize reproductive success, many animals restrict breeding to a given season when conditions are favourable (e.g. abundant food, low thermoregulatory demands) for successful rearing of offspring (Baker 1938; Bronson 1989) and curtail breeding during unfavourable conditions enabling greater energetic investment directed towards individual survival (e.g. cellular maintenance, thermoregulation or immune function; Demas & Nelson 1998; Demas 2004).
The neuroendocrine pathway regulating reproductive status is the hypothalamo–pituitary–gonadal (HPG) axis. Reproductive activity is induced by the release of hypothalamic gonadotropin-releasing hormone (GnRH), and the subsequent release of pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH act on the gonads to induce the production of sex steroids and gamete development. Sex steroids, in turn, act on the brain to promote appropriate sexual behaviour.
Photoperiod (i.e. day length) acts as the major environmental signal cuing favourable environmental conditions for most temperate-zone seasonal breeders. However, short-term environmental challenges may arise (e.g. sudden winter storm in the late spring) and animals must either maintain or alter HPG axis activity appropriately in response to both long-term (e.g. photoperiod) and moment-to-moment changes in the environment (Wingfield et al. 1998). The effects of photoperiod on HPG axis activity have been well established in seasonal breeders (for review see Dawson et al. (2001) and Goldman (2001)); however, the neuroendocrine signals relaying photoperiod and additional environmental signals (e.g. food availability) to the HPG axis remain less well understood (Wingfield 2008).
Recently, a class of peptides with a common Arg-Phe-NH2 C-terminus (called RFamide peptides) has been shown to affect reproductive neuroendocrine activity (for reviews see Kriegsfeld 2006; Tsutsui & Ukena 2006). This class of peptides was initially shown to influence a number of physiological processes in invertebrates (Price & Greenberg 1977). More recently, several classes of RFamides have been isolated in vertebrates (figure 1); these peptides exert influence on a variety of physiological processes such as food intake, pain perception and endocrine activity (Tsutsui & Ukena 2006; Zajac & Mollereau 2006). Importantly for the study of reproduction, two of these RFamides, kisspeptin (also known as metastin) and GnIH (also known as RFamide-related peptide, RFRP), have emerged as major regulators of HPG axis activity (for reviews see Kriegsfeld 2006; Tsutsui & Ukena 2006).
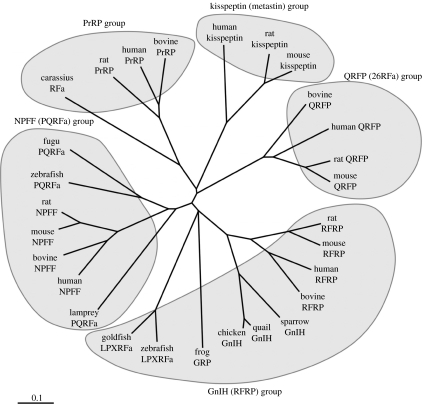
Figure 1.
Phylogenetic tree of the RFamide peptide family. To date, five groups of the RFamide peptide family have been documented: (i) NPFF (PQRFa) group, (ii) PrRP group, (iii) GnIH (RFRP) group, (iv) kisspeptin (metastin) group, and (v) QRFP (26RFa) group. GnIH and mammalian GnIH homologous peptides inhibit gonadotropin secretion and share a common C-terminal LPXRFamide (X is L or Q) motif. By contrast, the newly identified neuropeptide kisspeptin (metastin) acts to stimulate the reproductive axis. Mammalian kisspeptin possesses a C-terminal RFamide or RYamide motif. (See Ukena & Tsutsui 2005; Tsutsui & Ukena 2006; Osugi et al. 2006).
Kisspeptin, the peptide encoded by the KiSS-1 gene, plays an important role in the development and upregulation of the reproductive system (de Roux et al. 2003; Funes et al. 2003; Seminara et al. 2003), whereas GnIH has been reported to play an important role in regulating reproduction by downregulating release of pituitary gonadotropins via actions on the HPG axis (Tsutsui et al. 2000; Ciccone et al. 2004; Osugi et al. 2004; Bentley et al. 2006b; Kriegsfeld et al. 2006; Ubuka et al. 2006; Johnson et al. 2007). The distinct opposing roles played by these two peptides, along with associated changes in the predicted direction following photoperiodic manipulations (described below), suggest that these peptides may provide key modulatory input necessary for precise control of reproductive activity in seasonal breeders.
2. Kisspeptin actions on the HPG axis
Although only recently characterized, a great deal of progress has been made in uncovering the mechanisms through which kisspeptin acts to stimulate the reproductive axis. Kisspeptin neurons are distributed across several tissues (reviewed in Kauffman et al. 2007a), but importantly for reproduction, they are localized to at least two discrete regions in the hypothalamus in rodents, the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei (reviewed in Smith & Clarke 2007). Neurons from these nuclei send projections to the medial preoptic area (POA), a brain region containing GnRH cell bodies (Hahn & Coen 2006), although it is not yet known whether kisspeptin neurons specifically contribute to these projections. The receptor for kisspeptin, G-protein-coupled receptor 54 (GPR54), has been localized to a majority of GnRH neurons (Irwig et al. 2004; Messager et al. 2005; Quaynor et al. 2007) and a number of lines of evidence indicate that kisspeptin produces its effects via actions on GnRH neurons (reviewed in Smith & Clarke 2007). Despite the apparent presence of GPR54 in the pituitary gland (Kotani et al. 2001; Muir et al. 2001), examination of the effects of kisspeptin on gonadotropin release in pituitary cells in vitro has yielded equivocal results (Matsui et al. 2004; Thompson et al. 2004; Navarro et al. 2005; Gutierrez-Pascual et al. 2007), whereas in vivo examination of the effects of kisspeptin on the pituitary has failed to elicit gonadotropin release when the receptor for GnRH is blocked pharmacologically (Gottsch et al. 2004; Irwig et al. 2004; Shahab et al. 2005; Plant et al. 2006; Mason et al. 2007b). These data suggest a direct action of kisspeptin at the level of the hypothalamus, with a limited or no role in regulating HPG axis activity at the level of the pituitary.
Both Kiss-1 and GPR54 mRNAs have been localized to the ovaries and the testes of rodents (Kotani et al. 2001; Ohtaki et al. 2001; Terao et al. 2004; Castellano et al. 2006a), and a functional kisspeptin–GPR54 system may provide a mechanism for local regulation of reproductive status at the level of the gonads, although this is at present only speculative.
3. Gonadotropin-inhibitory hormone actions on the HPG axis
GnIH received its name owing to its inhibitory actions on pituitary gonadotropin release in avian species (Tsutsui et al. 2000). More recent evidence indicates that similar mechanisms of pituitary control exist across mammals (Clarke et al. in review). GnIH cell bodies are primarily localized to the paraventricular nucleus (PVN) in birds (e.g. sparrows, quail and starlings; Tsutsui et al. 2000; Ubuka et al. 2003, 2008; Ukena et al. 2003; Osugi et al. 2004) and the dorsomedial nucleus of the hypothalamus (DMH) in rodents (Kriegsfeld et al. 2006; Johnson et al. 2007). Fibres, presumably from the cell bodies localized in the PVN (birds) and DMH (rodents), extend to the median eminence and the hypothalamus, and caudally through the brain to at least the brain stem (reviewed in Bentley et al. 2006a). The GnIH receptor is a G-protein-coupled receptor (Yin et al. 2005) and mRNA for this peptide has been localized to the hypothalamus (both GnRH-I and -II neurons), pituitary, testes and epididymides in quail and the testes, ovaries and oviduct in European starlings (Yin et al. 2005; Bentley et al. 2008; Ubuka et al. 2008) as well as to gonadotropes in the quail pituitary (V. S. Chowdhury, T. Ubuka, G. E. Bentley & K. Tsutsui 2008, unpublished data). These data, combined with functional studies, indicate that, like kisspeptin, GnIH is able to alter activity of the HPG axis via direct actions on GnRH neurons (Bentley et al. 2003, 2008; Kriegsfeld et al. 2006; Ubuka et al. 2008). Unlike kisspeptin, GnIH is also able to alter pituitary gonadotropin release directly (Tsutsui et al. 2000; Osugi et al. 2004). The functional role of GnIH and its receptor in the gonads remains uncertain (Bentley et al. 2008).
4. Kisspeptin and GnIH as neuroendocrine integrators of photoperiodic cues
(a) Photoperiodic influences on kisspeptin
In seasonally breeding rodents, specifically Siberian (Phodopus sungorus) and Syrian hamsters (Mesocricetus auratus), photoperiod-driven changes in reproduction are associated with marked changes in both KiSS-1 mRNA (Syrian hamster) and kisspeptin (Siberian hamster) in the brain (Revel et al. 2006; Greives et al. 2007; Mason et al. 2007b). Additionally, acute administration of exogenous kisspeptin stimulates the HPG axis in all seasonally breeding mammals studied to date (Messager et al. 2005; Revel et al. 2006; Caraty et al. 2007; Greives et al. 2007; Mason et al. 2007b); however the effect of prolonged kisspeptin treatment on the HPG axis appears inconsistent. In Siberian hamsters, six weeks of daily kisspeptin injections were unable to alter gonadal state (Greives et al. 2008), and continuous kisspeptin (i.e. four weeks) administration to Siberian hamsters with regressed gonads held in ‘winter-like’ photoperiod did not lead to an elevation of LH or gonadal recrudescence (Greives et al. 2008). In one account using male Syrian hamsters, continuous release of kisspeptin across four weeks induced gonadal recrudescence in animals with regressed reproductive systems (Revel et al. 2006). The data from Syrian hamsters are at odds with findings in mature rats and rhesus monkeys where continuous kisspeptin exposure caused desensitization of GnRH neurons and testicular degeneration (Seminara et al. 2006; Thompson et al. 2006; Ramaswamy et al. 2007; Roa et al. 2008). Further, in anoestrous sheep, continuous kisspeptin infusion initially elevated GnRH and LH levels, but after 2 h the concentrations of these molecules displayed a continuous decline (Messager et al. 2005), and after 48 hours of infusion, LH levels did not differ from those of vehicle-treated sheep (Caraty et al. 2007). Based on these findings, we hypothesize that chronic kisspeptin treatment may desensitize GnRH neurons in hamsters, as has been suggested by previous studies in rats and monkeys (Seminara et al. 2006; Thompson et al. 2006). The cause of the differential response to prolonged kisspeptin exposure observed in Syrian hamsters compared with other mammalian species is not known but may be the result of different dosage regimes or routes of administration. A more likely alternative is that a fundamental difference between species exists (e.g. site of melatonin action, see below), and further investigating the response to prolonged kisspeptin may provide a unique opportunity to investigate evolutionary patterns leading to differences in response to this peptide.
Whereas the brain areas in which kisspeptin expression is observed are consistent across many rodent species, the direction of change in kisspeptin expression in response to changing photoperiod differs across taxa. In Siberian hamsters, immunohistochemistry reveals greater AVPV kisspeptin immunoreactivity in animals held in reproductively stimulating long days compared with reproductively inhibitory short days (figure 2). By contrast, kisspeptin immunoreactivity in the ARC is relatively low in long days, while being significantly greater in short days (Greives et al. 2007; Mason et al. 2007b). In Syrian hamsters, however, ARC KiSS-1 mRNA increases in hamsters held in stimulatory long days and decreases in non-reproductive hamsters; KiSS-1 expression was not observed in the AVPV (Revel et al. 2006), a region where kisspeptin is seen in other rodent species (Smith et al. 2005; Greives et al. 2007; Kauffman et al. 2007b). It remains unclear what role kisspeptin neurons in each of these nuclei play in regulating HPG axis responses. It will be important to deduce not only the relative role these areas of the brain play in regulating reproduction, but also to understand how they respond to neuroendocrine signals relaying environmental (e.g. photoperiod) and internal information (e.g. melatonin, sex steroids; Smith et al. 2005). Discerning how and why differences in expression and action of similar mechanisms among seasonally breeding species arise may help us understand how certain selective pressures drive divergent patterns of regulatory mechanisms across a wide range of habitats and breeding pressures.
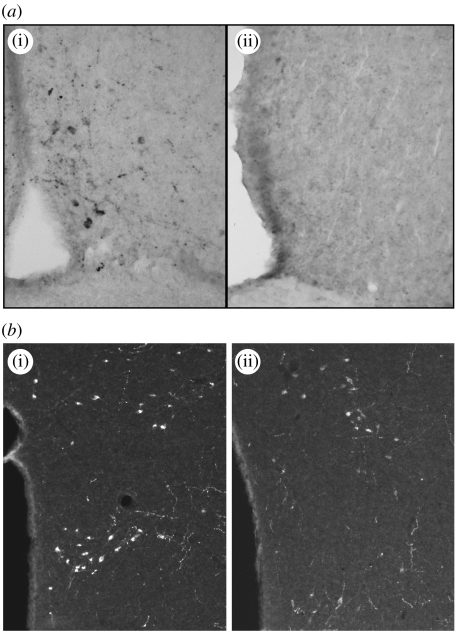
Figure 2.
Kisspeptin and GnIH response to photoperiod. Photoperiod alters kisspeptin immunoreactivity in Siberian hamsters, with (a(i)) long-day housed animals displaying significantly more kisspeptin-ir cells in the anteroventral periventricular nucleus compared with (a(ii)) short-day housed animals. Photoperiod also alters GnIH in the dorsomedial hypothalamus of Syrian hamsters with (b(ii)) short-day housed animals displaying significantly fewer GnIH-ir neurons, compared with (b(i)) long-day housed animals. (Adapted from Greives et al. (2007) and Mason et al. (2007a)).
Photoperiodic (i.e. day length) information in mammals is coded into a biochemical signal via melatonin, an indoleamine hormone released from the pineal primarily during darkness. The duration of melatonin secretion serves as a physiological signal for day length and is responsible for alteration of HPG axis activity in photoperiodic mammals (Carter & Goldman 1983; Bartness et al. 1993). Observed photoperiod-induced changes in the kisspeptin system, combined with evidence that pinealectomy alters KiSS-1 expression (Revel et al. 2006), suggest responsiveness of the kisspeptin system to melatonin; however, in the absence of additional data, this idea is speculative at present. Interestingly, the sites of melatonin action responsible for alteration of reproductive status differ in the Syrian and Siberian hamster brains (Goldman 2001), and uncovering the relationship between kisspeptin and melatonin in these species may help to understand not only the observed differences in responses to kisspeptin and its expression patterns in response to photoperiod, but also uncover common themes for how actions of the same molecule may diverge across species.
The vast majority of studies to date on the kisspeptin system have been in mammals. More recently, investigations into non-mammalian species have uncovered a functional kisspeptin–GPR54 system in fish (Kanda et al. 2008; van Aerle et al. 2008); however, preliminary attempts to uncover a functional kisspeptin-GPR54 system in birds have thus far proved to be relatively unfruitful (O'Brien et al. 2005; S. E. Schrock, L. J. Kriegsfeld, G. E. Demas & E. D. Ketterson 2007, unpublished data). Overcoming the challenges associated with developing species-specific investigative tools (e.g. antibodies) will be necessary to enable comparative studies of this peptide.
(b) Photoperiodic influences on GnIH
GnIH, like kisspeptin, displays changes in the pattern of expression of mRNA and peptide in response to changing photoperiod and reproductive status in seasonally breeding birds. Female song sparrows display increased GnIH immunoreactivity when becoming photorefractory to long days (i.e. termination of the breeding season; Bentley et al. 2003). Although the role of melatonin in regulating mammalian seasonal reproduction has long been known (Bartness et al. 1993), traditionally it was not thought to play an active role in avian reproductive cycles (Dawson et al. 2001). Contrary to this traditional view, a recent finding reported that administration of melatonin alters GnIH content in quail (Ubuka et al. 2005), while a separate study of castrated white leghorn roosters receiving injections of relatively high doses of melatonin displayed up to 70% reductions in plasma LH (Rozenboim et al. 2002). These findings suggest a potential mode for melatonin to modulate the avian HPG axis indirectly through the GnIH system.
Mammalian GnIH (RFRP) has been identified and localized to the DMH in the seasonally breeding Syrian hamster (Kriegsfeld et al. 2006). The DMH is a site of high-density melatonin binding and this brain region is critical for appropriate gonadal involution in response to short-day photoperiods (Maywood et al. 1996). Thus, localization to the DMH indicates the possibility that photoperiod may play a key role in altering GnIH expression. Only very recently has the response of GnIH to photoperiod manipulation been investigated in mammals. Both Syrian and Siberian hamsters display significant changes in GnIH-like content in response to photoperiod manipulation (Mason et al. 2007a; Revel et al. 2008). Given its role in inhibiting HPG activity, it was expected that inhibitory (i.e. short-day) photoperiods would stimulate an upregulation of GnIH in the DMH. However, in these studies GnIH-like immunoreactivity and gene expression in rodents were lower in animals held on short-day photoperiods (Mason et al. 2007a; Revel et al. 2008; figure 2). Further investigations aimed at uncovering the role mammalian DMH GnIH neurons play in the regulation of the HPG activity, as well as the temporal pattern of GnIH expression in response to short-day photoperiods, are needed.
5. Kisspeptin and GnIH: a yin–yang relationship?
The opposing effects of these peptides in seasonally breeding species suggest that they may interact to provide a mechanism to coordinate reproductive responses precisely to environmental variables, although other peptides are likely to be involved in this process as well. In temperate environments, increasing day length provides an initial predictive cue, indicating a higher probability of favourable environmental conditions (Wingfield 1980, 1983; Wingfield & Farner 1993). However, local environmental conditions may vary from year to year, thus necessitating the use of additional cues to fine-tune the exact timing of mating (Wingfield et al. 1992; Wingfield & Farner 1993; Perfito et al. 2004). The potential ability for kisspeptin and GnIH to respond to multiple relevant stimuli and exert influence at multiple levels of the HPG axis offers an exciting prospect to provide a mechanism for more precise control or modulation of the timing of breeding (figure 3).
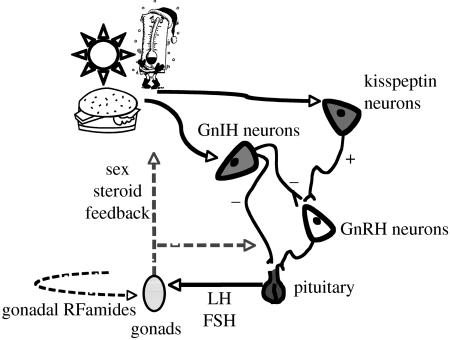
Figure 3.
Hypothetical model of kisspeptin and GnIH action. In this model, we hypothesize that the production of kisspeptin and GnIH in the brain is altered in response to relevant environmental cues. Evidence indicates that photoperiodic cues act on the system via changes in melatonin signalling. Hormonal signals of energy reserves, such as leptin, also appear able to alter these molecules. In a hypothetical scenario, in favourable environments kisspeptin has a stimulatory effect on GnRH neurons leading to the release of GnRH and an upregulation of the HPG axis, while in unfavourable conditions GnIH would downregulate the HPG axis at the level of GnRH neurons and/or the level of the pituitary. In addition both kisspeptin and GnIH may alter reproductive functionality at the level of the gonads via endocrine and/or paracrine actions.
In our theoretical model, kisspeptin might respond to a shortening melatonin signal associated with lengthening photoperiods (Bartness et al. 1993; Revel et al. 2006), stimulating the HPG axis via activation of GnRH neurons. However, in the event of inclement weather or a sudden reduction in resources, reproduction may be inhibited or delayed (e.g. Wingfield 1985a,b). Here, GnIH may temporarily override the activation of the HPG axis through inhibitory actions at the level of the pituitary and/or GnRH neurons (figure 3). Although the functional roles of kisspeptin and GnIH at the level of the gonads are as of yet unknown, they may provide an additional level of regulatory control on reproductive physiology in response to environmental signals altering circulating (i.e. endocrine) or local (i.e. paracrine) secretion of these peptides. Uncovering how these peptidergic systems respond moment to moment to relevant signals at multiple levels in the HPG axis will further elucidate their role in allowing for precisely timed reproductive events (Wingfield 2008).
6. Future directions
While considerable progress has been made in elucidating the roles that kisspeptin and GnIH play in reproduction, our understanding of their role in seasonal reproduction is in its infancy. Non-photoperiodic cues that vary seasonally (i.e. supplementary cues), such as food availability (Bronson 1989) and/or energy stores, may play an important role in fine-tuning the expression and interaction of these peptides (as described previously); however, the effects of these cues have not yet been investigated in a seasonal context. The kisspeptin system in non-seasonal laboratory rodents is altered in response to changes in energetic resources (e.g. fasting) or signals of energy availability (e.g. leptin; Castellano et al. 2005, 2006b; Smith et al. 2006; Luque et al. 2007). Recent evidence suggests that GnIH may interact with the ingestive system in chickens and rats, with injections of GnIH leading to a significant increase in feeding behaviour (Tachibana et al. 2005; Johnson et al. 2007). Thus, it is likely that seasonal breeders respond not only to photoperiodic cues, but also to supplementary cues that allow for adjustments of the kisspeptin and GnIH systems in response to real-time changes in energy availability. Additionally, these data suggest the intriguing idea that RFamides may be able to transmit information of ‘reproductive status’ to systems controlling other seasonally relevant, non-reproductive behaviours such as food intake and energy metabolism. One recent report suggests no observable change in hypothalamic GnIH content in response to moderate food restriction in broiler breeder hens (Ciccone et al. 2007); however, the lack of observable changes in this study should be interpreted with caution, as these animals have experienced artificial selection under ideal food conditions. Investigations aimed at uncovering the effects of food and other environmental cues (e.g. temperature, water availability) in non-domesticated animals will provide insights into the potential interactions between these cues and GnIH and kisspeptin.
Temperate-zone animals with longer gestation/incubation periods than those of rodents or birds also must time periods of fertility to facilitate offspring birth in the favourable conditions of spring/early summer. For example, seasonally breeding sheep, with a considerably longer gestation period compared with rodents, initiate reproductive behaviours and fertility during short days (e.g. autumn and winter; reviewed in Malpaux et al. 2001). Recent studies in ovariectomized sheep indicate an association between the short-day reproductive season and kisspeptin expression, with an increase of KiSS-1 mRNA in the ARC from the transition from long-day photoperiods to fertile short-day photoperiods; a similar, but non-significant, increase was observed in the POA (Smith et al. 2007). Additionally, kisspeptin infusion initially activates the HPG axis in female acyclic sheep, inducing ovulation; however, exposure to kisspeptin in these sheep over a prolonged period downregulates the HPG axis (i.e. LH levels return to baseline; Caraty et al. 2007). The role GnIH plays in short-day breeders has not yet been investigated.
Non-seasonal breeders (i.e. flexibly breeding species) must also ensure that reproduction occurs during favourable times. In these animals, swift and precise alteration of the HPG axis may be beneficial. Investigations of kisspeptin and GnIH in these species may yield insights into the versatility and flexibility these peptides may provide to allow for non-seasonal breeding. Recently, it has been reported that in the flexibly breeding Rufous-winged sparrow, exogenous GnIH is unable to reduce LH levels during the breeding period associated with the monsoons of the Sonoran desert (Deviche et al. 2006). Exploring the possible role kisspeptin may play in this and similar species both during the breeding and non-breeding periods may help clarify the relationship between GnIH and kisspeptin.
Lastly, the sexes of the same species may differ in the maintenance or activation of reproductive behaviour and physiology (e.g. Beery et al. 2007) and provide a unique avenue for investigation of these peptides within the same species. Female Siberian hamsters, unlike males, fail to elevate LH levels in response to exogenous kisspeptin when housed in inhibitory short-day photoperiods (Mason et al. 2007b; figure 4); females in this species must carry offspring to term and provide unaided care (Wynne-Edwards 1995). In this instance, GnIH may act to provide a sex-specific mechanism as a ‘fail-safe’ switch, ensuring avoidance of the energetic cost associated with activation of the HPG axis in response to kisspeptin and avert a potential mistimed pregnancy, although currently this idea remains highly speculative. Thus far, sex differences in GnIH have not been reported, and future research will be needed to adequately test these and other hypotheses.
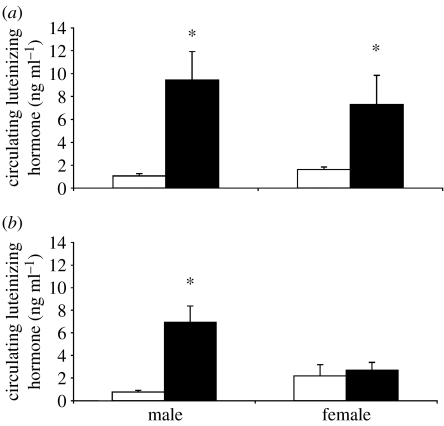
Figure 4.
Sex differences in kisspeptin responses. (a) Circulating levels of LHs are elevated in long-day housed male and female Siberian hamsters after the administration of kisspeptin. (b) Following kisspeptin injection, short-day housed (non-reproductive) male Siberian hamsters significantly elevate circulating LH levels, while short-day housed (non-reproductive) female Siberian hamsters fail to display a significant elevation in circulating levels of LH, indicating potential sex differences (Adapted from Greives et al. (2007) and Mason et al. (2007b)). White bar, baseline LH; black bar, post-injection LH.
7. Conclusion
Although many insights have been gained in the few years since the discovery of kisspeptin and GnIH, many questions remain unanswered. Both peptides appear to respond to a variety of environmental signals; however, it is unknown whether these peptides respond similarly or whether one peptide is affected more robustly than the other in response to these cues. Studies conducted under controlled laboratory and semi-naturalistic environments, combined with field investigations, will be needed to gain a greater understanding of the role that these peptides play in regulating ecologically relevant reproductive responses. In addition, comparative investigations focusing on the natural history, ecology and environmental factors affecting a wide range of vertebrates will be necessary to uncover the ability of kisspeptin and GnIH to enable appropriate alterations of reproductive status in animals occupying divergent habitats. Substantial opportunities exist to examine how evolution has shaped these systems or how variation in these systems may have allowed selection pressures to shape the neural mechanisms driving reproduction in disparate habitats.
Acknowledgments
The authors would especially like to thank A. Mason, M. Scotti and E. Ketterson for their invaluable help, discussion and encouragement along the way and two anonymous referees whose constructive critique improved the quality and clarity of the current manuscript. We would also like to thank D. Zysling, E. Chester, C. Bergeon, J. Ho, J. Levine, S. Frommeyer and J. Lodde for assistance and discussion. This work has been supported by an SICB Grant-in-Aid and NIH/T32 HD 049336 (T.J.G.), NSF IOB 0543798 and an Indiana University Faculty Research Support Program (G.E.D.), NSF IOS 0641188 and UC Berkeley Committee on research grant (G.E.B.) and an NIH HD 050470 and UC Berkeley Committee on research grant (L.J.K.). Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (16086206 and 18107002 to K.T.)
References
- Baker J.R. The evolution of breeding seasons. In: DeBeer G.B, editor. Evolution: essays on aspects of evolutionary biology. Clarendon Press; Oxford, UK: 1938. pp. 161–177. [Google Scholar]
- Bartness T.J, Powers J.B, Hastings M.H, Bittman E.L, Goldman B.D. The timed infusion paradigm for melatonin delivery—what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses. J. Pin. Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. doi:10.1111/j.1600-079X.1993.tb00903.x [DOI] [PubMed] [Google Scholar]
- Beery A.K, Trumbull J.J, Tsao J.M, Costantini R.M, Zucker I. Sex differences in the onset of seasonal reproductive quiescence in hamsters. Proc. R. Soc. B. 2007;274:281–286. doi: 10.1098/rspb.2006.3726. doi:10.1098/rspb.2006.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley G.E, Perfito N, Ukena K, Tsutsui K, Wingfield J.C. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J. Neuroendocrinol. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Kriegsfeld L.J, Osugi T, Ukena K, O'Brien S, Perfito N, Moore I.T, Tsutsui K, Wingfield J.C. Interactions of gonadotropin-releasing hormone (GnRH) and gonadotropin-inhibitory hormone (GnIH) in birds and mammals. J. Exp. Zool. Part A-Comp. Exp. Biol. 2006a;305:807–814. doi: 10.1002/jez.a.306. doi:10.1002/jez.a.306 [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Perfito N, Moore I.T, Ukena K, Tsutsui K, Wingfield J.C. Gonadotropin-inhibitory hormone in birds: possible modes of action. Acta Zool. Sin. 2006b;52(Suppl.):178–182. [Google Scholar]
- Bentley G.E, et al. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen. Comp. Endocrinol. 2008;156:34–43. doi: 10.1016/j.ygcen.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bronson F.H. University of Chicago Press; Chicago, IL: 1989. Mammalian reproductive biology. [Google Scholar]
- Caraty A, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258. doi: 10.1210/en.2007-0554. doi:10.1210/en.2007-0554 [DOI] [PubMed] [Google Scholar]
- Carter D.S, Goldman B.D. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Castellano J.M, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. doi:10.1210/en.2005-0337 [DOI] [PubMed] [Google Scholar]
- Castellano J.M, et al. Expression of KiSS-1 in rat ovary: putative local regulator ovulation? Endocrinology. 2006a;147:4852–4862. doi: 10.1210/en.2006-0117. doi:10.1210/en.2006-0117 [DOI] [PubMed] [Google Scholar]
- Castellano J.M, et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006b;55:2602–2610. doi: 10.2337/db05-1584. doi:10.2337/db05-1584 [DOI] [PubMed] [Google Scholar]
- Ciccone N.A, Dunn I.C, Boswell T, Tsutsui K, Ubuka T, Ukena K, Sharp P.J. Gonadotrophin inhibitory hormone depresses gonadotrophin alpha and follicle-stimulating hormone beta subunit expression in the pituitary of the domestic chicken. J. Neuroendocrinol. 2004;16:999–1006. doi: 10.1111/j.1365-2826.2005.01260.x. doi:10.1111/j.1365-2826.2005.01260.x [DOI] [PubMed] [Google Scholar]
- Ciccone N.A, Dunn I.C, Sharp P.J. Increased food intake stimulates GnRH-L glycoprotein hormone alpha-subunit and follistatin mRNAs, and ovarian follicular numbers in laying broiler breeder hens. Domest. Anim. Endocrinol. 2007;33:62–76. doi: 10.1016/j.domaniend.2006.04.008. doi:10.1016/j.domaniend.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. et al In Review. Evidence of an hypophysiotropic role for gonadotropin-inhibitory hormone.
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Demas G.E. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm. Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. doi:10.1016/j.yhbeh.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Demas G.E, Nelson R.J. Photoperiod, ambient temperature, and food availability interact to affect reproductive and immune function in adult male deer mice (Peromyscus maniculatus) J. Biol. Rhythms. 1998;13:253–262. doi: 10.1177/074873098129000093. doi:10.1177/074873098129000093 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel J.C, Matsuda F, Chaussain J.L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl Acad. Sci. USA. 2003;100:10 972–10 976. doi: 10.1073/pnas.1834399100. doi:10.1073/pnas.1834399100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deviche P, Small T, Sharp P, Tsutsui K. Control of luteinizing hormone and testosterone secretion in a flexibly breeding male passerine, the Rufous-winged sparrow, Aimophila carpalis. Gen. Comp. Endocrinol. 2006;149:226–235. doi: 10.1016/j.ygcen.2006.06.004. doi:10.1016/j.ygcen.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick J.A, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S.J, Monsma F.J, Gustafson E.L. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biophys. Res. Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. doi:10.1016/j.bbrc.2003.11.066 [DOI] [PubMed] [Google Scholar]
- Goldman B.D. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. doi:10.1177/074873001129001980 [DOI] [PubMed] [Google Scholar]
- Gottsch M.L, Cunningham M.J, Smith J.T, Popa S.M, Acohido B.V, Crowley W.F, Seminara S, Clifton D.K, Steiner R.A. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. doi:10.1210/en.2004-0431 [DOI] [PubMed] [Google Scholar]
- Greives T.J, Mason A.O, Scotti M.A.L, Levine J, Ketterson E.D, Kriegsfeld L.J, Demas G.E. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. doi:10.1210/en.2006-1249 [DOI] [PubMed] [Google Scholar]
- Greives T.J, Kriegsfeld L.J, Demas G.E. Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus) Gen. Comp. Endocrinol. 2008;156:552–558. doi: 10.1016/j.ygcen.2008.02.017. doi:10.1016/j.ygcen.2008.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Pascual E, Martinez-Fuentes A.J, Pinilla L, Tena-Sempere M, Malagon M.M, Castano J.P. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J. Neuroendocrinol. 2007;19:521–530. doi: 10.1111/j.1365-2826.2007.01558.x. doi:10.1111/j.1365-2826.2007.01558.x [DOI] [PubMed] [Google Scholar]
- Hahn J.D, Coen C.W. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J. Comp. Neurol. 2006;494:190–214. doi: 10.1002/cne.20803. doi:10.1002/cne.20803 [DOI] [PubMed] [Google Scholar]
- Irwig M.S, Fraleyb G.S, Smith J.T, Acohido B.V, Popa S.M, Cunningham M.J, Gottsch M.L, Clifton D.K, Steiner R.A. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. doi:10.1159/000083140 [DOI] [PubMed] [Google Scholar]
- Johnson M.A, Tsutsui K, Fraley G.S. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. doi:10.1016/j.yhbeh.2006.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda S, Akazome Y, Matsunaga T, Yamamoto N, Yamada S, Tsukamura H, Maeda K.I, Oka Y. Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually-dimorphic kisspeptin neurons in Medaka (Oryzias latipes) Endocrinology. 2008;149:2467–2476. doi: 10.1210/en.2007-1503. doi:10.1210/en.2007-1503 [DOI] [PubMed] [Google Scholar]
- Kauffman A.S, Clifton D.K, Steiner R.A. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007a;30:504–511. doi: 10.1016/j.tins.2007.08.001. doi:10.1016/j.tins.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Kauffman A.S, Gottsch M.L, Roa J, Byquist A.C, Crown A, Clifton D.K, Hoffman G.E, Steiner R.A, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007b;148:1774–1783. doi: 10.1210/en.2006-1540. doi:10.1210/en.2006-1540 [DOI] [PubMed] [Google Scholar]
- Kotani M, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001;276:34 631–34 636. doi: 10.1074/jbc.M104847200. doi:10.1074/jbc.M104847200 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L.J. Driving reproduction: RFamide peptides behind the wheel. Horm. Behav. 2006;50:655–666. doi: 10.1016/j.yhbeh.2006.06.004. doi:10.1016/j.yhbeh.2006.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld L.J, Mei D.F, Bentley G.E, Ubuka T, Mason A.O, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc. Natl Acad. Sci. USA. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. doi:10.1073/pnas.0511003103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque R.M, Kineman R.D, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. doi:10.1210/en.2007-0500 [DOI] [PubMed] [Google Scholar]
- Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythms. 2001;16:336–347. doi: 10.1177/074873001129002051. doi:10.1177/074873001129002051 [DOI] [PubMed] [Google Scholar]
- Mason, A. O., Duffy, S. P., Bentley, G. E., Ubuka, T., Tsutsui, K., Silver, R. & Kriegsfeld, L. J. 2007a Expression of gonadotropin-inhibitory hormone is influenced by reproductive condition independently of photoperiod. Program No. 831.8. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007. Online.
- Mason A.O, Greives T.J, Scotti M.L, Levine J, Frommeyer S, Ketterson E.D, Demas G.E, Kriegsfeld L.J. Supression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lenghts in female Siberian hamsters. Horm. Behav. 2007b;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. doi:10.1016/j.yhbeh.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem. Biophys. Res. Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. doi:10.1016/j.bbrc.2004.05.185 [DOI] [PubMed] [Google Scholar]
- Maywood E.S, Bittman E.L, Hastings M.H. Lesions of the melatonin-and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol. Reprod. 1996;54:470–477. doi: 10.1095/biolreprod54.2.470. doi:10.1095/biolreprod54.2.470 [DOI] [PubMed] [Google Scholar]
- Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl Acad. Sci. USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. doi:10.1073/pnas.0409330102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A.I, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 2001;276:28 969–28 975. doi: 10.1074/jbc.M102743200. doi:10.1074/jbc.M102743200 [DOI] [PubMed] [Google Scholar]
- Navarro V.M, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. doi:10.1210/en.2004-0836 [DOI] [PubMed] [Google Scholar]
- O'Brien S, Bentley G.E, Wingfield J.C. KiSS increases LH in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii) Integr. Comp. Biol. 2005;45:1173. [Google Scholar]
- Ohtaki T, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. doi:10.1038/35079135 [DOI] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Bentley G.E, O'Brien S, Moore I.T, Wingfield J.C, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J. Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. doi:10.1677/joe.0.1820033 [DOI] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Sower S.A, Kawauchi H, Tsutsui K. Evolutionary origin and divergence of PQRFamide peptides and LPXRFamide peptides in the RFamide peptide family. Insights from novel lamprey RFamide peptides. FEBS J. 2006;273:1731–1743. doi: 10.1111/j.1742-4658.2006.05187.x. doi:10.1111/j.1742-4658.2006.05187.x [DOI] [PubMed] [Google Scholar]
- Perfito N, Tramontin A.D, Meddle S, Sharp P, Afik D, Gee J, Ishii S, Kikuchi M, Wingfield J.C. Reproductive development according to elevation in a seasonally breeding male songbird. Oecologia. 2004;140:201–210. doi: 10.1007/s00442-004-1576-5. doi:10.1007/s00442-004-1576-5 [DOI] [PubMed] [Google Scholar]
- Plant T.M, Ramaswamy S, DiPietro M.J. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. doi:10.1210/en.2005-1261 [DOI] [PubMed] [Google Scholar]
- Price D.A, Greenberg M.J. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197:670–671. doi: 10.1126/science.877582. doi:10.1126/science.877582 [DOI] [PubMed] [Google Scholar]
- Quaynor S, Hu L, Leung P.K, Feng H, Mores N, Krsmanovic L.Z, Catt K.J. Expression of a functional G protein-coupled receptor 54-Kisspeptin autoregulatory system in hypothalamic gonadotropin-releasing hormone neurons. Mol. Endocrinol. 2007;21:3062–3070. doi: 10.1210/me.2007-0207. doi:10.1210/me.2007-0207 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara S.B, Pohl C.R, DiPietro M.J, Crowley W.F, Jr, Plant T.M. Effect of continuous intravenous administration of Human Metastin 45–54 on the neuroendocrine activity of the hypothalamic–pituitary–testicular axis in the adult male Rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–3370. doi: 10.1210/en.2007-0207. doi:10.1210/en.2007-0207 [DOI] [PubMed] [Google Scholar]
- Revel F.G, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen J.D, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr. Biol. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. doi:10.1016/j.cub.2006.07.025 [DOI] [PubMed] [Google Scholar]
- Revel F.G, Saboureau M, Pevet P, Simonneaux V, Mikkelsen J.D. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902–912. doi: 10.1210/en.2007-0848. doi:10.1210/en.2007-0848 [DOI] [PubMed] [Google Scholar]
- Roa, J. et al 2008 Desensitization of gonadotropin responses to Kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am. J. Physiol. Endocrinol. Metab (doi:10.1152/ajpendo.90240.2008) [DOI] [PubMed]
- Rozenboim I, Aharony T, Yahav S. The effect of melatonin administration on circulating plasma luteinizing hormone concentration in castrated White Leghorn roosters. Poult. Sci. 2002;81:1354–1359. doi: 10.1093/ps/81.9.1354. [DOI] [PubMed] [Google Scholar]
- Seminara S.B, et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. doi:10.1056/NEJMoa035322 [DOI] [PubMed] [Google Scholar]
- Seminara S.B, DiPietro M.J, Ramaswamy S, Crowley W.F, Plant T.M. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. doi:10.1210/en.2005-1550 [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara S.B, Crowley W.F, Ojeda S.R, Plant T.M. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc. Natl Acad. Sci. USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. doi:10.1073/pnas.0409822102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.T, Clarke I.J. Kisspeptin expression in the brain: catalyst for the initiation of puberty. Rev. Endocr. Metab. Disord. 2007;8:1–9. doi: 10.1007/s11154-007-9026-4. doi:10.1007/s11154-007-9026-4 [DOI] [PubMed] [Google Scholar]
- Smith J.T, Dungan H.M, Stoll E.A, Gottsch M.L, Braun R.E, Eacker S.M, Clifton D.K, Steiner R.A. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. doi:10.1210/en.2005-0323 [DOI] [PubMed] [Google Scholar]
- Smith J.T, Acohido B.V, Clifton D.K, Steiner R.A. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. doi:10.1111/j.1365-2826.2006.01417.x [DOI] [PubMed] [Google Scholar]
- Smith J.T, Clay C.M, Caraty A, Clarke I.J. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. doi:10.1210/en.2006-1435 [DOI] [PubMed] [Google Scholar]
- Tachibana T, Sato M, Takahashi H, Ukena K, Tsutsui K, Furuse M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005;1050:94–100. doi: 10.1016/j.brainres.2005.05.035. doi:10.1016/j.brainres.2005.05.035 [DOI] [PubMed] [Google Scholar]
- Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. BBA-Gene Struct. Expr. 2004;1678:102–110. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Thompson E.L, Patterson M, Murphy K.G, Smith K.L, Dhillo W.S, Todd J.F, Ghatei M.A, Bloom S.R. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic–pituitary–gonadal axis. J. Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. doi:10.1111/j.1365-2826.2004.01240.x [DOI] [PubMed] [Google Scholar]
- Thompson E.L, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am. J. Physiol. Endocrinol. Metab. 2006;291:E1074–E1082. doi: 10.1152/ajpendo.00040.2006. doi:10.1152/ajpendo.00040.2006 [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K. Review: hypothalamic LPXRF-amide peptides in vertebrates: identification, localization and hypophysiotropic activity. Peptides. 2006;27:1121–1129. doi: 10.1016/j.peptides.2005.06.036. doi:10.1016/j.peptides.2005.06.036 [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp J.P. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. doi:10.1006/bbrc.2000.3350 [DOI] [PubMed] [Google Scholar]
- Ubuka T, Ueno M, Ukena K, Tsutsui K. Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system. J. Endocrinol. 2003;178:311–318. doi: 10.1677/joe.0.1780311. doi:10.1677/joe.0.1780311 [DOI] [PubMed] [Google Scholar]
- Ubuka T, Bentley G.E, Ukena K, Wingfield J.C, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl Acad. Sci. USA. 2005;102:3052–3057. doi: 10.1073/pnas.0403840102. doi:10.1073/pnas.0403840102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Ukena K, Sharp P.J, Bentley G.E, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. doi:10.1210/en.2005-1178 [DOI] [PubMed] [Google Scholar]
- Ubuka T, Kim S, Huang Y.C, Reid J, Jiang J, Osugi T, Chowdhury V.S, Tsutsui K, Bentley G.E. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149:268–278. doi: 10.1210/en.2007-0983. doi:10.1210/en.2007-0983 [DOI] [PubMed] [Google Scholar]
- Ukena K, Tsutsui K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: structure, localization and function. Mass Spectrom. Rev. 2005;24:469–486. doi: 10.1002/mas.20031. doi:10.1002/mas.20031 [DOI] [PubMed] [Google Scholar]
- Ukena K, Ubuka T, Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003;312:73–79. doi: 10.1007/s00441-003-0700-x. doi:10.1007/s00441-003-0700-x [DOI] [PubMed] [Google Scholar]
- van Aerle R, Kille P, Lange A, Tyler C.R. Evidence for the existence of a functional Kiss1/Kiss1 receptor pathway in fish. Peptides. 2008;29:57–64. doi: 10.1016/j.peptides.2007.10.018. doi:10.1016/j.peptides.2007.10.018 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C. Fine temporal adjustment of reproductive functions. In: Epple A, Stetson M.H, editors. Avian endocrinology. Academic Press; New York, NY: 1980. pp. 367–389. [Google Scholar]
- Wingfield, J. C. 1983 Environmental and endocrine control of avian reprodution: an ecological approach. In Avian endocrinology (eds S. Mikami, K. Homma & M. Wada), pp. 265–288. Tokyo, Japan: Japan Scientific Societies Press; Berlin, Germany: Springer.
- Wingfield J.C. Influences of weather on reproductive function in female song sparrows, Melospiza melodia. J. Zool. (Lond.) 1985a;205:545–558. [Google Scholar]
- Wingfield J.C. Influences of weather on reproductive function in male song sparrows, Melospiza melodia. J. Zool. (Lond.) 1985b;205:525–544. [Google Scholar]
- Wingfield J.C. Organization of vertebrate annual cycles: implications for control mechanisms. Phil. Trans. R. Soc. B. 2008;363:425–441. doi: 10.1098/rstb.2007.2149. doi:10.1098/rstb.2007.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield, J. C. & Farner, D. S. 1993 Endocrinology of reproduction in wild species. In Avian biology, vol. 9 (eds D. S. Farner, J. R. King & K. C. Parkes), pp. 163–327. New York, NY: Academic Press.
- Wingfield J.C, Hahn T.P, Levin R, Honey P. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 1992;261:214–231. doi:10.1002/jez.1402610212 [Google Scholar]
- Wingfield J.C, Maney D.L, Breuner C.W, Jacobs J.D, Lynn S, Ramenofsky M, Richardson R.D. Ecological bases of hormone–behavior interactions: the “Emergency Life History Stage”. Am. Zool. 1998;38:191–206. [Google Scholar]
- Wynne-Edwards K.E. Biparental care in Djungarian but not Siberian dwarf hamsters (Phodopus) Anim. Behav. 1995;50:1571–1585. doi:10.1016/0003-3472(95)80012-3 [Google Scholar]
- Yin H, Ukena K, Ubuka T, Tsutsui K. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J. Endocrinol. 2005;184:257–266. doi: 10.1677/joe.1.05926. doi:10.1677/joe.1.05926 [DOI] [PubMed] [Google Scholar]
- Zajac J.M, Mollereau C. Special issue: RFamide peptides—introduction. Peptides. 2006;27:941–942. doi: 10.1016/j.peptides.2005.12.005. doi:10.1016/j.peptides.2005.12.005 [DOI] [PubMed] [Google Scholar]