Abstract
Chromatin remodeling by post-translational modification of histones plays an important role in brain plasticity, including memory, response to stress and depression. The importance of H3/4 histones acetylation by CREB binding protein (CBP) or related histone acetyltransferase, including p300, was specifically demonstrated using knockout (KO) mouse models. The physiological role of a related protein that also acts as a transcriptional coactivator with intrinsic histone acetylase activity, the p300/CBP associated factor (PCAF), is poorly documented. We analyzed the behavioral phenotype of homozygous male and female PCAF KO mice and report a marked impact of PCAF deletion on memory processes and stress response. PCAF KO animals showed short-term memory deficits at 2 months of age, measured using spontaneous alternation, object recognition or acquisition of a daily changing platform position in the water-maze. Acquisition of a fixed platform location was delayed, but preserved, and no passive avoidance deficit was noted. No gender-related difference was observed. These deficits were associated with hippocampal alterations in pyramidal cell layer organization, basal levels of Fos immunoreactivity and MAP kinase activation. PCAF KO mice also showed an exaggerated response to acute stress, forced swimming and conditioned fear, associated with increased plasma corticosterone levels. Moreover, learning and memory impairments worsened at 6 and 12 months of age, when animals failed to acquire the fixed platform location in the water-maze and showed passive avoidance deficits. These observations demonstrate that PCAF histone acetylase is involved lifelong in the chromatin remodeling necessary for memory formation and response to stress.
Keywords: histone acetylase PCAF, short-term memory, spatial memory, stress, age, behavioral phenotyping
INTRODUCTION
Post-translational histone modifications exert a regulatory role during gene transcription by remodeling the structure of chromatin DNA-protein complexes (Varga-Weisz, 2005). Acetylation of specific lysine residues on H3 or H4 histones generally allows binding of the transcriptional machinery to DNA and facilitating transcription (Jenuwein and Allis, 2001). Conversely, H3 or H4 histone methylation promotes transcriptional repression (Lachner et al, 2001). Histone methylation may also facilitate DNA methylation, which in turn induces further transcriptional repression(Lachner et al, 2001). Chromatin remodeling plays an important role in several aspects of long-term cellular plasticity, including neuronal differentiation (Lunyak et al, 2002), seizures (Tsankova et al, 2004), learning and memory (Korzus et al, 2004; Levenson et al, 2004; Levenson and Sweatt, 2005), responses to stress and depression (Tsankova et al, 2006), drug addiction (Kumar et al, 2005) and neurodegeneration (Steffan et al, 2001; Hoshino et al, 2003). In particular, a direct effect of fear conditioning consolidation, and subsequent activation of N-methyl-D-aspartate (NMDA) receptors increased histone H3 acetylation in the CA1 area of the hippocampus, and this effect was blocked by inhibition of ERK signaling (Levenson et al, 2004). Moreover, chronic social defeat stress increased repressive histone methylation and subsequent treatment with the tricyclic antidepressant imipramine reversed this downregulation and increased histone acetylation (Tsankova et al, 2006). Histones post-translational modifications, leading to chromatin remodeling, therefore appears to be a very rapid regulatory system for brain plasticity.
Other regulators of gene expression include transcription factors, such as the cAMP-responsive element binding protein (CREB) or its coactivator, the CREB binding protein (CBP) (Chan and La Thangue, 2006). However, CBP, and its homolog p300, also acts as a scaffold protein by interacting with several transcription factors and components of the RNA polymerase II (Pol II) and as a histone acetyltransferase (Ogryzko et al, 1996; Janknecht, 2002; Chan and La Thangue, 2006). Both functions are intimately linked and dynamic changes in chromatin organization directly affect gene expression. The importance of CBP and/or its histone acetyltransferase activity in memory consolidation was recently demonstrated using either heterozygous CBP knockout (KO) mice (Oike et al, 1999; Alarcon et al, 2004) or a mouse expressing a dominant negative CBP{HAT−} variant, in which the acetyltransferase activity is abolished (Korzus et al, 2004).
A unique role, although not yet documented, could also be played by the p300/CBP-associated factor (PCAF), which also acts as a transcriptional coactivator bearing intrinsic histone acetylase activity. PCAF substrates include histones (H3 on Lys14 and H4 on Lys9), coactivators and transcription factors (Imhof et al, 1997; Munshi et al, 1998; Liu et al, 1999; Sartolli et al, 1999; Martinez-Balbas et al, 2000). PCAF is present in a large multiprotein complex consisting of more than 20 distinct polypeptides that are able to acetylate histones in a nucleosomal context (Ogryzko et al, 1998). PCAF belongs to the Gcn5 related N-acetyl transferase (GNAT) super family of acetyltransferase and shares close similarity with GCN5 over its entire sequence. Both proteins are present in the same complexes and GCN5 can act at the same Lys on histones H3 and H4 as PCAF, suggesting that both acetylases may have redundant biological activity.
Homozygous PCAF KO and GCN5 KO mice have been generated by replacing part of the PCAF or GCN5 gene with a neo gene cassette using homologous recombination (Yamauchi et al, 2000). Unexpectedly, these mice showed distinct phenotypes. The GCN5 homozygous KO was lethal in embryo, while PCAF KO mice displayed no obvious phenotype (Yamauchi et al, 2000). GCN5 is an essential gene expressed ubiquitously and early during development, i.e. by day 8 post-coitum (dpc). PCAF, however, is expressed later in embryonic development at 12.5 dpc, and is not essential for viability. Moreover, in early embryogenesis, PCAF and GCN5 are differentially regulated and may have distinct biological activity. Particular, transcriptional activation by PCAF is required for nuclear receptor-mediated activation, the growth factor-signaled pathway and interferon activation (Blanco et al, 1998; Xu et al, 1998; Masumi et al, 1999).
In the present study, the involvement of PCAF in learning and memory processes and stress response was examined using the PCAF KO mouse. Young 2 month old male and female PCAF KO mice were examined through a battery of behavioral tests to assess short- and long-term, spatial, contextual and recognition learning for memory ability and response to acute stress or haedonia for emotional response. The behavioral phenotyping was sustained by morphological and physiological examination of the hippocampal formation and stress hormone assays.
MATERIALS AND METHODS
Animals
The generation of PCAF KO mice and subsequent backcross of the CD-1 strain was previously described (Yamauchi et al, 2000). PCAF KO and wild-type (WT) littermate mice were housed in groups of ten, allowed food ad libitum, and maintained in a controlled environment (22 ± 1°C, 55 ± 5% humidity) with a 12 h:12 h light:dark cycle. Animals care was in accordance with the European Community Council Directive of 24 Nov. 1986 (86-609). Male mice were used at 2- and 6 months of age and female mice were used at 2- and 12 months of age. Mice genotypes were obtained by PCR on genomic DNA from tail tips using specific primers amplifying WT (forward 5′ TTC TAG ATC TGC CGG TGT CC 3′, reverse: 5′ CTG CCA GAC CCT GTT TAC AC 3′), which detect the wild-type allele, and Neo (forward: 5′ TCG CCT TCT TGA CGA GTT CT 3′, reverse: 5′ GGC TGC TGA TCT CGT TCT TC 3′) DNA sequences, which detect the neomycin cassette.
Biochemical analyses
Protein extractions
Mice were sacrificed by decapitation, the brain removed and the hippocampi dissected out on ice and immediately frozen by immersion in liquid nitrogen. For cytoplasmic proteins analysis, frozen samples were boiled during 5 min in 100 μl of 2% sodium dodecylsulfate (Sigma-Aldrich, France) after sonication. After centrifugation, 10 min at 20,000 g, supernatants were recolted and protein concentration was determined with a protein assay kit, using bovine serum albumin as standard (Biorad, France). For histone extraction, all procedures were performed on ice and all solutions were cooled to 4°C before to use. All centrifugation steps were performed at 4°C. Frozen samples were incubated for 10 min in 4ml of homogenization buffer containing in mM: 250 sucrose, 50 Tris-HCl pH 7.5, 25 KCl, 0.5 phenylmethylsulfonylfluoride, 0.9 sodium butyrate and protease inhibitor cocktail (Roche, France). They were then homogenized with a Dounce glass homogenizer for 14 strokes. Tissue homogenates were centrifuged at 7,700 g for 1 min. The cytosolic fraction (supernatant) was aspired out and stored at −80 °C. Histones were acid extracted from the nuclear fraction (pellet) in 1 ml of 0.4 N H2SO4 for 30 min. Acid extracts were centrifuged at 14,000 g for 10 min. Histones (supernatant) were transferred to a fresh tube and precipitated with addition of trichloroacetic acid containing 4 mg/ml sodium deoxycholate (Sigma-Aldrich) for 30 min. Precipitated proteins were collected by centrifugation at 14,000 g for 30 min. The supernatant was discarded and the protein pellet was washed with 1 ml of acidified acetone (0.1% HCl) for 5 min. The proteins were collected with a centrifugation at 14,000 g for 5 min and then washed a second time with 1 ml of acetone for 5 min. A final centrifugation at 14,000 g for 5 min was performed to collect the proteins and the supernatant was discarded. The resulting purified proteins were resuspended in 10 mM Tris pH 8 and stored at −80°C. Protein concentration was measured using the BCA Protein Assay Kit (Pierce, France).
Proteins analysis by Western blotting
Proteins (50 μg for phospho-Erk1/2, 4 μg for histone) were diluted in an equal volume of Laemmli buffer, boiled for 5 min and loaded on a SDS-polyacrylamid gel at 12% for phospho-Erk1/2 and16.5% for histone. The resolved proteins were transferred to a nitrocellulose blotting membrane. The membrane was then blocked during 30 min at room temperature with 5% non-fat dry milk in Tris-buffered saline 20 mM pH 7.6 containing 0.1% Tween-20 (TBS-T) for phospho-Erk1/2. The membrane was incubated overnight at 4 °C, rinsed for 30 min in TBS-T and then incubated for 2 h with a horseradish peroxidase-conjugated secondary antibody for phospho-Erk1/2. For histone H3 acetylation analysis, all procedures were performed according to manufacturer’s recommendation. Peroxidase activity was revealed by using enhanced chemiluminescence (ECL) reagents. Then, intensity of peroxidase activity was semi-quantified using the Image J® software. Results were normalized to control values (anti-MAP Kinase 1/2 for phospho-Erk1/2, gels stained with Coomassie blue for histone).
Antibodies
The primary antibodies used, and their dilutions were as follows: anti phospho-MAP Kinase 1/2 (#07-467, Upstate Biotechnology, NY, USA, dilution 1:500), anti MAP Kinase 1/2 (#06-182, Upstate Biotechnology, dilution 1:1000) and anti acetyl-Histone H3 (Lys-14, #06-599, Upstate Biotechnology, dilution 1:1000). The secondary antibody was horseradish peroxidase-conjugated Goat anti-rabbit IGg (Sigma-Aldrich, dilution 1:2000).
Morphological analyses
Cresyl violet staining
Each mouse was anaesthetized with sodium pentobarbital (100 mg/kg i.p.) and quickly transcardially perfused with 50 ml of phosphate buffered saline solution, pH = 7.2, followed by 50 ml of phosphate buffered saline solution containing 4% paraformaldehyde (w/v). Brains were removed and kept overnight in the fixative solution. They were cut in coronal sections (50 μm thickness) using a vibratome (Leica VT1000 S). Serial sections were selected to include the hippocampal formation and placed in gelatin-coated slides. Sections were stained with 0.2% Cresyl violet reagent (Sigma-Aldrich, France), then dehydrated with graded ethanol, treated with xylene and mounted with DePeX medium (BDH Laboratory Supplies, England). Examination of the CA1 area was performed using a light microscope (Dialux 22, Leica, Germany), and the slices were digitalized through a CCD camera (Sony XC-77CE, Japan) with the NIH Image v1.63 software, in order to facilitate processing of cell layer thickness measurements and pyramidal cells counts. Three sub-regions were examined, i.e. CA1, CA3 and the dentate gyrus. The median width was calculated from three measures on one slice and three to eight slices were counted per region per mouse. The results are presented as mean ± S.E.M. and analyzed using the Student’s t-test.
Fos protein immunohistochemistry
After transcardiac perfusion with paraformaldehyde 4% and 24 h post fixation, coronal sections (50 μm thickness) were cut to include the rostral part of the hippocampus and incubated for 10 min with 1% H2O2 to inhibit endogenous peroxidase activity, and then with a Fos antibody (SC-52, Santa Cruz, CA, USA; dilution 1:5000) for 48 h in a phosphate buffer solution containing 2% bovine serum albumin (Sigma-Aldrich) and 0.1% Triton X-100 (Sigma-Aldrich), and then with a peroxidase-conjugated goat anti-rabbit IgG for 2 h (Jackson ImmunoResearch Europe, U.K.; dilution 1:500). Slices were rinsed at each step with PBS, mounted after dehydratation and observed under a light microscope. Fos immunostaining was measured with a microcomputer imaging device (SAMBA® analysis Software, Alcatel, France) capturing the image through a CCD camera connected to a light microscope (Axioskop, Zeiss, Germany). Immunoreactive cells were detected and standardized via NIH image© analysis. The results are presented as mean ± S.E.M. and analyzed using Newman-Keuls’ test.
Plasma corticosterone assay
Plasma corticosterone was assayed, as previously reported (Givalois et al, 2004), with a radioimmunoassay kit (Biotrak, Amersham Bioscience, Saclay, France) in a 50 μl plasma sample diluted (1:5) with the assay buffer and heated to 60°C for 30 min to displace corticosterone to the cortisol binding protein according to the manufacturer’s instructions. The intra- and inter-assay coefficients of variation were 5% and 7%, respectively. The assay sensitivity was 0.6 ng/ml. The results are presented as mean ± S.E.M. and analyzed using Newman-Keuls’ test.
Behavioral testing
Open-field behavior
The general motility of mice was examined in a circular wooden arena (diameter 75 cm). Two concentric circles were drawn on the floor (diameter 15 cm and 45 cm, resp.), with the outer ring being divided into 8 partitions and the middle ring into 4 partitions. The open-field session consisted of placing the mouse in the center circle and monitoring its movements for 10 min using a video camera. The following parameters were evaluated: (1) the time taken to move out of the center circle; (2) locomotion activity, in terms of number of partitions crossed, then calculated as the distance traveled (m); (3) immobility duration; (4) locomotor speed; (5) locomotion activity in the five central partitions; (6) rearing frequency; (7) grooming frequency; and (8) number of defecations. The results were analyzed at 2 months of age using two-way ANOVA (F value), with substrain and gender as independent factors, group comparisons using the Newman-Keuls’ test and the Student’s t-test for the groups at 6 or 12 months of age.
Spatial learning in the water-maze
The water-maze was a circular pool (diameter 170 cm, height 40 cm). The water temperature, 23 ± 1°C, was set to avoid animal hypothermia. Light intensity, external cues in the room, and water opacity (obtained by suspension of lime carbonate) were rigorously reproduced. A transparent plexiglas non-slippery platform (diameter 10 cm) was immersed 2 cm under the water surface during acquisition trials. Swimming was recorded using Videotrack® (version NT) software (Viewpoint, Champagne-au-Mont-d’Or, France), with trajectories being analyzed as latencies and distances. The software divided the pool into four quadrants. Training consisted of three swims per day for 7 days (9 for 12-m.o. mice), with 15 min intertrial time. Start positions, set at each limit between quadrants, were randomly selected for each animal. Each animal was allowed a 90 s swim to find the platform and was left for a further 20 s on the platform. The median latency, expressed as mean ± S.E.M., was calculated for each training day and analyzed over trials using a repeated-measures ANOVA test (F values) for the latencies and Fisher’s exact test for the avoidance percentages. Retention data were analyzed using Dunn’s test after Kruskal-Wallis ANOVA (KW values).
A probe test was performed 1 h after the last swim on day 7 or 9. The platform was removed and each animal was allowed a free 60 s swim. The start position for each mouse corresponded to one of two positions remote from the platform location in counterbalanced order. The platform quadrant was termed the training (T) quadrant and others opposite (O), adjacent right (AR), and adjacent left (AL) during retention. The percentage of time spent in each quadrant was determined.
A procedure for specifically assessing the short-term memory component was then performed for 3 days. The platform location changed every day but not among trials. Start positions, set at each limit between quadrants, were randomly selected for each animal. Each animal was allowed a 90 s swim to find the platform and was left for a further 20 s on the platform. The animal was allowed 5 trials per day, with a 2 min intertrial time interval. Data represent the mean performance over days for each trial. Escape latencies were analyzed using the Friedman non-parametric repeated-measures ANOVA, with group comparisons made using Dunn’s test.
Spontaneous alternation in the Y-maze
The maze was made of black painted wood. Each arm was 40 cm long, 13 cm high, 3 cm wide at the bottom, 10 cm wide at the top, and converging at an equal angle. Each mouse was placed at the end of one arm and allowed to move freely through the maze during an 8 min session. The series of arm entries, including possible returns into the same arm, was recorded using an Apple IIe computer. An alternation was defined as entries into all three arms on consecutive occasions. The number of maximum alternations was therefore the total number of arm entries minus two and the percentage of alternation was calculated as (actual alternations/maximum alternations) × 100. The data were analyzed using two-way ANOVA (F value), with substrain and gender as independent factors, and group comparisons made using Newman-Keuls’ test.
Object recognition procedure
The apparatus consisted of a squared open-field (1 × 1 × 0.4 m high), made of dark gray plastic. Lighting was provided indirectly by a 60 W bulb positioned 2 m above the floor. The open-field was surrounded by white curtains (2 m high) in order to provide a visually uniform experimental environment, except for a striped pattern (29 cm wide, 21 cm high), consisting of an alternation of 2.5 cm wide black and white vertical bars, attached on the wall opposite to the position where the animals were initially placed. A CCD camera above the field was connected to a video tracking system (Videotrack®, ViewPoint, Champagne-au-Mont-d’Or, France). Objects could be placed at four defined positions, which differed by their shape and texture: (A) a black plastic chair foot cover; (B) a circular plastic box; (C) a transparent glass 25-ml erlen vial; (D) a metallic toy battery; and (E) a plastic 50 ml vial (Falcon). Animals’ movements were quantified by video tracking, in terms of distance and duration within different areas. The software highlighted four circular areas around the object positions and two square areas dividing the open-field in different alleys, i.e. the outer close to the walls, the inner at the center of the field and the intermediate including the object positions. An experienced experimenter quantified the number of contacts with objects, defined as the mouse’s snout actually touching the object. Mice were submitted individually to 7 successive 6-min sessions, with an inter-trial time interval of 3 min. The sequence for the successive sessions was as follows: session 1, the open-field was empty and animals were allowed a free exploration session in order to evaluate their locomotor activity, reaction to novelty and anxiety; sessions 2—4: the four objects A—D were placed in the open-field and exploratory behavior and object habituation were assessed through quantification of contacts with objects and locomotor activity; session 5—6, objects C and D were inverted in order to evaluate the reaction to spatial change; session 7, a novel object (E) replaced a familiar non-displaced object (A) in order to measure the reaction to novelty, object recognition and/or neophobia. The starting position was unchanged throughout the sessions, and animals were tested in a semi-randomized order. The arena and objects were cleaned using a 1:1 ethanol:water solution between each session. Data were analyzed using multiple comparison one-way or two-way ANOVA, using session, object, gender or substrain as independent factors, as specified. ANOVA was followed by the Newman-Keuls’ post-hoc comparison test.
Step-down type passive avoidance test
The apparatus consisted of a transparent acrylic cage (30 × 30 × 40 cm high) with a grid-floor, inserted in a soundproof outer box (35 × 35 × 90 cm high). The cage was illuminated with a 15 W lamp during the experimental period. A wooden platform (4 × 4 × 4 cm) was fixed at the centre of the grid-floor. Electric shocks (1 Hz, 500 ms, 43 V DC) were delivered to the grid-floor using an isolated pulse stimulator (Model 2100, AM Systems, Everett, WA, USA). The test involved two training sessions, at 90-min time interval, and in a retention session carried out 24 h after the first training. Each mouse was placed on the platform during the training sessions. When it stepped down and placed its four paws on the grid-floor, shocks were delivered for 15 s. Step-down latency and the numbers of vocalizations and flinching reactions were measured. Shock sensitivity was evaluated by summing these last two numbers. None of the treatments used in the present study significantly affected the shock sensitivity. Animals which did not step down within 60 s during the second session were considered as remembering the task and taken off without receiving any more electric shocks. The retention test was performed in a similar manner as training, except that the shocks were not applied to the grid-floor. Each mouse was placed again on the platform, and the latency was recorded, with an upper cut-off time of 300 s. Two parametric measures of retention were analyzed: the latency and the number of animals reaching the avoidance criterion, defined as correct if the latency measured during the retention session was greater than 3-fold the latency shown by the animal during the second training session and at least greater than 60 s. Latencies did not show a normal distribution, since cut-off times were set, and were thus expressed as median and interquartile range. They were analyzed using the Kruskal-Wallis non-parametric analysis of variance (KW values), with group comparisons being made with the Dunn’s non-parametric multiple comparisons test.
Forced swimming test
Each mouse was placed in a glass cylinder (diameter 12 cm, height 24 cm) filled with water at a height of 12 cm. Water temperature was maintained at 22–23°C. The animal was forced to swim for 15 min on the first day. The session was videotaped and the duration of immobility during the first 6 min was analyzed minute per minute. On the second day, each mouse was placed again in the water and forced to swim for 6 min. The session was videotaped and the duration of immobility was analyzed minute per minute. The mouse was considered as immobile when it stopped struggling and moved only to remain floating in the water. Results were analyzed using multiple comparison two-way ANOVA, using time (min or day) and substrain as independent factors, followed by a Newman-Keuls’ post-hoc comparison test.
Conditioned fear stress
The apparatus was a transparent acrylic rectangular cage (30 × 30 × 40 high cm) equipped with a metal wire floor. On day 1, each mouse was placed in the test cage and received intermittent electric shocks (0.1 Hz, 200 ms, 60 V DC) for 10 min through an isolated pulse stimulator (Model 2100, AM-Systems, Everett, WA, USA). When the mouse was placed in the test cage, the current resistance ranged from 100 to 250 kOhm. Therefore each mouse received electric shocks ranging from 0.24 to 0.60 mA. The test session was performed 24 h after the first session. Animals were placed again in the test cage, but no foot shock was delivered. The ambulatory activity of mice during each session was measured for 6 min using an infrared beam activity device (Opto-varimex, Columbus Instruments, Columbus, OH, USA), in which the cage was inserted. The non-shocked control groups were operated similarly, except for the absence of shock treatment. Results were analyzed using multiple comparison two-way ANOVA, with time (min or day) and substrain as independent factors, followed by a Newman-Keuls’ post-hoc comparison test.
Measure of sucrose preference (anhaedonia)
Animals were placed in cages of four individuals 1 week before the test and were water deprived 16 h before each test. They were presented two weighted bottles of fluid, one with tap water, the other with a sucrose 4% solution, for 2 h. The bottles were weighed after 1 h and at the end of the test to evaluate the mean fluid intake per hour per mouse. They were then allowed free access to tap water for 6 h. The results showed that the contribution of the second hour could be neglected and therefore only the first hour consumption is presented. The test was performed daily for 9 days. Data were analyzed using multiple comparison two-way ANOVA, using day and substrain as independent factors.
RESULTS
Brain PCAF expression and histone acetylation in PCAF KO mice
The absence of expression of PCAF was initially checked by genotyping. This was carried out on DNA extracted from mouse tail (Fig. 1A). Real-time RT-PCR analyses from brain and hippocampal extracts confirmed the absence of PCAF expression in KO mice (Data not shown). We also examined the level of acetylation of histone H3 on Lys14, known as the target of PCAF, in the hippocampus. The level of histone H3 acetylation was not diminished in PCAF KO mice compared to WT mice (Fig. 1B).
Figure 1.
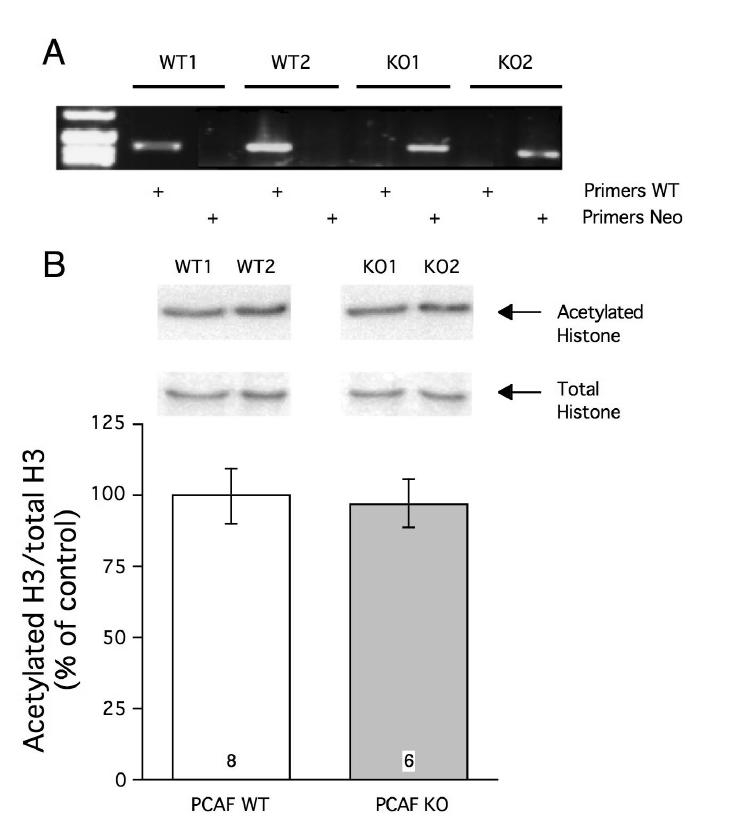
Absence of PCAF expression in PCAF KO mice, shown by q-PCR analyses (A); and histone H3 acetylation in PCAF KO mice (B). Representative q-PCR analyses and immunoblots for Lys14-acetylated H3 and total H3 are shown. Quantification of the immunoblot densities for Lys14-acetylated histone levels compared to total histone levels was performed on the number of animals indicated within the columns.
Short-term memory impairment in PCAF KO mice at 2 months of age
Prior to any behavioral investigation, we examined the general motility of female and male WT and PCAF KO mice in an open-field paradigm. As summarized in Table 1, two-way ANOVA analyses, with gender and substrain as independent factors, revealed no significant variations between wild-type and PCAF KO animals for any of the parameters examined. These observations concerning locomotion, exploration or anxiety behaviors indicated that the animals did not show any general behavioral deficits which would have impeded the validity of the memory tests.
Table 1.
Behavioral parameters of 2 month old female and male PCAF KO and WT mice in the open-field test
| Parameter | Female
|
Male
|
||
|---|---|---|---|---|
| WT | PCAF KO | WT | PCAF KO | |
| Weight (g) | 22.7 ± 0.9 | 22.0 ± 0.6 | 29.1 ± 0.9 | 27.9 ± 1.1 |
| Latency to start (s) | 5 ± 2 | 10 ± 3 | 15 ± 5 | 16 ± 4 |
| Locomotion (m) | 17.2 ± 1.6 | 13.8 ± 2.1 | 19.6 ± 2.9 | 18.3 ± 3.2 |
| Loc. in center (%) | 15.5 ± 3.9 | 9.1 ± 2.6 | 15.3 ± 2.7 | 18.0 ± 4.8 |
| Speed (m/min) | 2.56 ± 0.13 | 2.10 ± 0.17 | 2. 66 ± 0.31 | 2.54 ± 0.25 |
| Immobility (s) | 196 ± 20 | 210 ± 34 | 139 ± 25 | 146 ± 38 |
| Rearing | 10 ± 3 | 8 ± 3 | 15 ± 6 | 15 ± 5 |
| Grooming | 6 ± 1 | 5 ± 1 | 6 ± 1 | 5 ± 1 |
| Defecations | 4 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 |
| n | 7 | 8 | 9 | 8 |
Results were analyzed using a two-way ANOVA, with substrain and gender as independent factors and no behavioral parameters showed significant variations.
We then analyzed the behavioral consequences of inactivation of PCAF expression in a series of memory tasks involving spatial and associative learning. Female and male animals were examined in parallel.
Hippocampus-dependent learning abilities were examined using the Morris water-maze task. Animals were first submitted to repeated acquisition of a fixed platform location, i.e. a procedure involving short-term and reference memory components. For both PCAF KO and wild-type female mice, latencies in finding the platform decreased over the acquisition training period. The statistical analysis showed significant effects in WT mice (F(6,62) = 7.70, p < 0.0001) and PCAF KO mice (F(6,62) = 5.50, p < 0.001; Fig. 2A). Latencies shown by PCAF KO animals were augmented as compared with wild-type animals and significant differences were measured on days 2 and 4 (Fig. 2A). During the probe test, performed 1 h after the last trial, both wild-type and PCAF KO animals swam preferentially in the T quadrant during the 60-s session (F(3,35) = 7.67, p < 0.001 for female wild-type; F(3,35) = 13.80, p < 0.0001, for female PCAF KO mice; Fig. 2B). The time spent in this quadrant was similar between both groups, representing about 41%, indicating that they both correctly learned the platform location to an equal extent.
Figure 2.
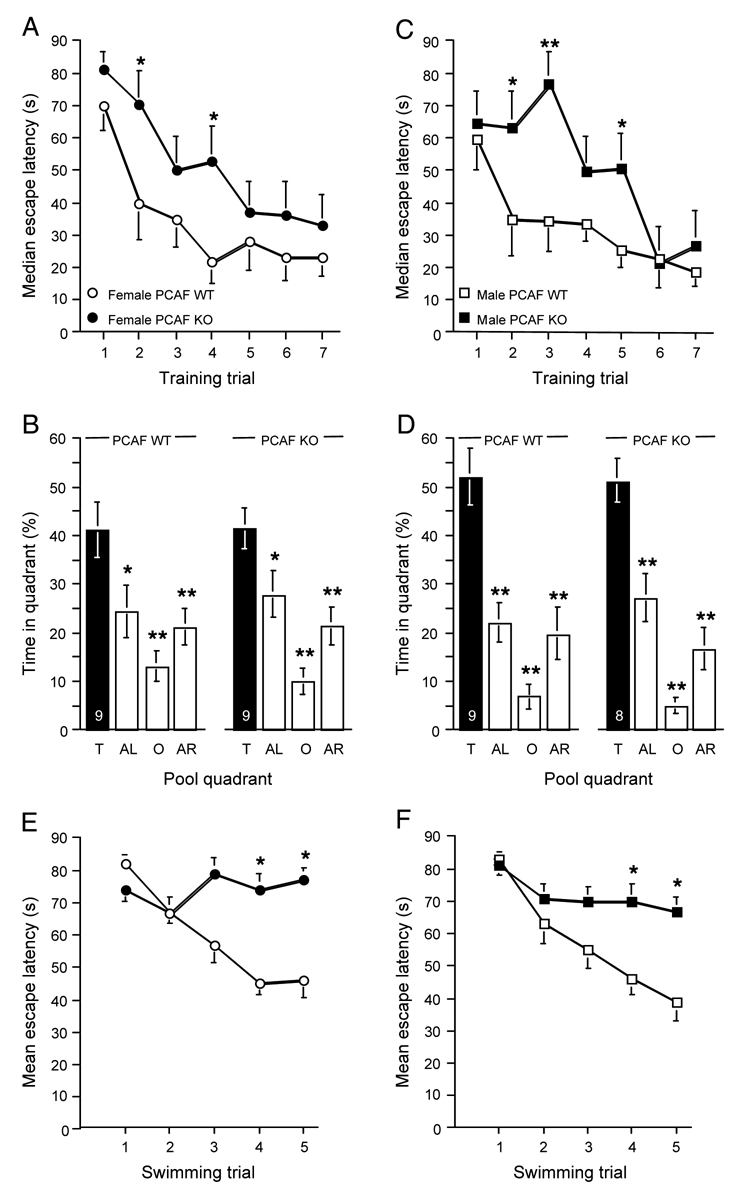
Impairment of spatial memory in PCAF KO mice. 2-month old female (left panel) and male (right panel) WT and PCAF KO mice acquired a fixed platform position in the water-maze test: acquisition profiles showing swimming duration (A, C); probe test performances (B, D). Animals were submitted for 7 days to 3 swims per day, with a 10 min intertrial time interval. Acquisition profiles are shown in terms of median swimming latency (A, C). * p < 0.05, ** p < 0.01 vs. the control value during the same training trial (Dunnett’s test). One hour after the last swimming trial, the platform was removed and animals were submitted to a 60 s swim. The presence in each quadrant was measured. Quadrants: T, training; AL, adjacent left; O, opposite; AR, adjacent right. The number of animals per group is indicated within the T column. * p < 0.05, ** p < 0.01 vs. the time spent in the T quadrant (Dunnett’s test). The short-term memory component was measured in the water-maze using a daily changing platform position procedure: swimming latency profiles among the five trials, averaged over the 3 days, for female (E) and male (F) WT and PCAF KO mice. * p < 0.05 vs. the control value (Dunn’s test).
Male animals also showed correct learning abilities, since latencies to find the platform decreased over the acquisition training period for wild-type mice (F(6,62) = 3.60, p < 0.01) or PCAF KO mice (F(6,55) = 6.56, p < 0.0001; Fig. 2C). Latencies shown by PCAF KO animals appeared to be higher than that observed for WT, with significant differences measured during days 2, 3 and 5 (Fig. 2C). During the probe test, both wild-type and PCAF KO mice swam preferentially in the T quadrant during the 60-s session (F(3,35) = 20.27, p < 0.0001 for WT; F(3,31)= 28.86, p < 0.0001, for PCAF KO; Fig. 2D). The time spent in this quadrant was similar between both groups, representing about 51%, indicating that they both correctly learned the location of the hidden platform to an equal extent.
Swimming speeds were calculated among groups and were: 29.4 ± 1.2 cm/s for female wild-type, 26.9 ± 0.9 cm/s for female PCAF KO, 25.5 ± 1.5 cm/s for male wild-type and 23.3 ± 1.2 cm/s for male PCAF KO. The two-way ANOVA indicated a significant effect for gender (F(1,31) = 9.18, p < 0.01) but not for substrain (F(1,31) = 3.62, p > 0.05) or gender × substrain (F(1,31) = 0.01, p > 0.05).
The spatial short-term memory was then estimated by changing the platform location daily for 3 training days. Each trial was an informative sample trial wherein the animal was allowed to swim to the platform at its new location. As shown in Fig. 2E, 2F, mice exhibited a progressive decrease in escape latency over the five trials. This decrease assessed the quality of spatial short-term memory. Female wild-type mice showed a significant decrease in escape latencies over the swimming trials (Fr = 17.35, p < 0.01; Fig. 2E). In particular, the latencies showed during trials 4 and 5 were significantly lower as compared to the 1st trial. Female PCAF KO mice failed to show a significant decrease in latency (Fr = 4.10, p > 0.05; Fig. 2E). During trials 4 and 5, the latencies measured for PCAF KO animals were significantly higher as compared to control animals. Male WT mice showed a significant decrease in escape latencies over the swimming trials (Fr = 14.42, p < 0.01; Fig. 2F). In particular, the latencies showed during trials 3 to 5 were significantly lower as compared to the 1st trial. Male PCAF KO mice failed to show a significant decrease in latency (Fr = 3.25, p > 0.05; Fig. 2F). The latency measured during the last two trials for PCAF KO animals was significantly higher as compared to wild-type animals.
Spatial working memory was examined using the spontaneous alternation procedure in the Y-maze, a non-aversive task allowing to differentiate between mnesic and locomotor behaviors. The substrain × gender analysis of the alternation percentage (Fig. 3A) showed significant effects for substrain (F(1,30) = 52.11, p < 0.0001), but not for gender (F(1,30) = 0.03, p > 0.05) or substrain × gender (F(1,30) = 1.12, p > 0.05). Post-hoc comparisons revealed highly significant impairment of the alternation percentage of PCAF KO animals. In terms of locomotion, assessed through the number of arms entered during the session (Fig. 3B), no significant effect was observed (F(1,30) = 0.39, p > 0.05 for substrain; F(1,30) = 1.52, p > 0.05 for gender; F(1,30) = 0.34, p > 0.05 for substrain × gender).
Figure 3.
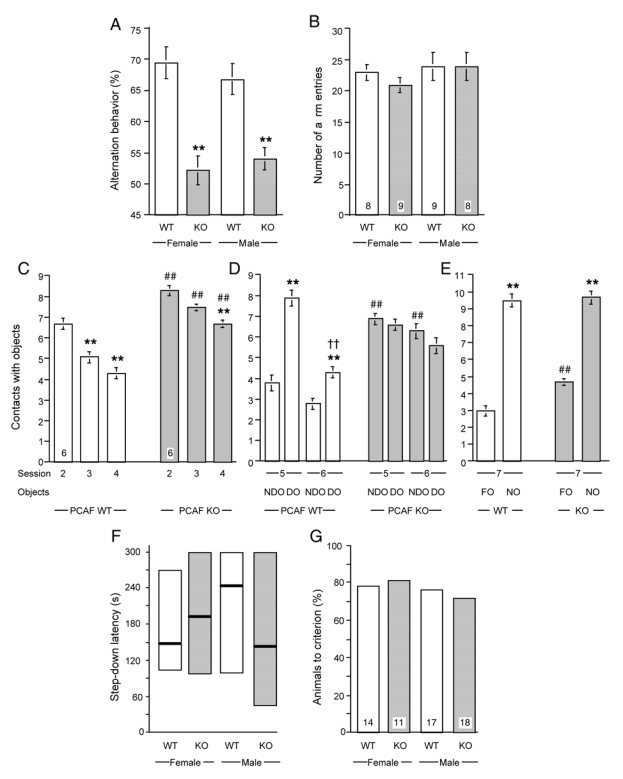
Impairment of spontaneous alternation, object recognition, and passive avoidance, in PCAF KO mice. Responses of 2 month old WT and PCAF KO mice in the spontaneous alternation test in the Y-maze: A, alternation percentage; B, total number of arms entered. Responses in the object recognition test: C, habituation to object exploration during sessions 2—4; D, reaction to change in object position during sessions 5, 6; E, reaction to a novel object during session 7. Responses in the passive avoidance test: F, step-down latency; G, percentage of animals to criterion. The number of animals per group is indicated within columns in B, C, G. Abbreviations: NDO, non-displaced objects; DO, displaced objects; FO, familiar objects; NO, novel object. Data were analyzed using a two-way ANOVA with substrain and gender as independent factors in A, B, F, G and substrain and sessions or objects as factors in C—E. ** p < 0.01 vs. the respective control value (Newman-Keuls’ test).
Male PCAF KO mice were tested for object recognition memory. Object-oriented exploratory activity and habituation was examined between sessions 2 and 4 by measuring the contact with four objects placed within the arena (Fig. 3C). The two-way ANOVA analysis revealed a significant effect for sessions (F(2,138) = 17.28, p < 0.0001) and substrain (F(1,138) = 61.23, p < 0.0001), but not for the interaction. PCAF KO mice showed a significantly higher level of interaction with objects for each session as compared with wild-type controls, but habituation persisted throughout the sessions (Fig. 3C), with a significant difference between session 2 and 4. The reaction to spatial change was examined by inverting the place of two objects before session 5, and measuring the contacts with objects between non-displaced and displaced objects during sessions 5 and 6 (Fig. 3D). ANOVA analyses were performed using displaced vs. non-displaced objects, with sessions and group treatments as independent factors. Significant effects were measured for object (F(1,87) = 9.94, p < 0.01), session (F(1,87) = 27.80, p < 0.0001), and substrain (F(1,87) = 21.53, p < 0.0001), but also for the object × session interaction (F(1,87) = 4.16, p < 0.05), object × substrain (F(1,87) = 22.70, p < 0.0001), and session × substrain (F(1,87) = 56.86, p < 0.05). Post-hoc comparisons showed that the control group presented preferential exploration of displaced objects as compared with non-displaced objects during session 5 and session 6, with significant habituation for the displaced object exploration during the last session. PCAF KO animals showed a higher level of interaction with non-displaced objects and no difference between displaced and non-displaced objects or between sessions (Fig. 3D). Reaction to novelty was assessed by exploration of a novel object during session 7 (Fig. 3E). A significant effect was observed for object (F(1,44) = 175.0, p < 0.0001), substrain (F(1,44) = 4.45, p < 0.05), but not for the object × substrain interaction. Control animals showed a highly significant preferential exploration of the novel object, and this trend also seemed to apply to PCAF KO animals, even though a higher level of contact with familiar objects was still measured (Fig. 3E).
Associative long-term memory was examined using a step-down type passive avoidance task. Two memory parameters were quantified, i.e. step-down latency and the percentage of animals reaching the avoidance criterion. The statistical analyses failed to show any significant effect for latency (KW = 0.59, p > 0.05; Fig. 3F) or the percentages of animals-to-criterion (Fig. 3G).
Hippocampal morphology in PCAF KO mice
An examination of the brain morphology was conducted using Cresyl violet staining. In particular, the pyramidal neuronal CA1/3 layers and the dendate gyrus in the hippocampal formation were analyzed, as detailed in Figure 3. Male PCAF KO mice, at 2 months of age, showed subtle modifications in hippocampal formation as compared with WT animals (Fig. 4A, 4B). In particular, we observed, in the pyramidal neuronal layer in the CA1 area, a moderate but significant decrease in the cell number and an increase in the layer thickness, as shown for typical slices in Fig. 4C, 4D and averaged in Fig. 4E. This indicated a decrease in cell density. In the CA3 area, a similar trend was observed (Fig. 4F, 4G), but no significant difference was measured (Fig. 4H). The morphological aspect of the dendate gyrus was similar between WT and PCAF KO animals (Fig. 4I–4K).
Figure 4.
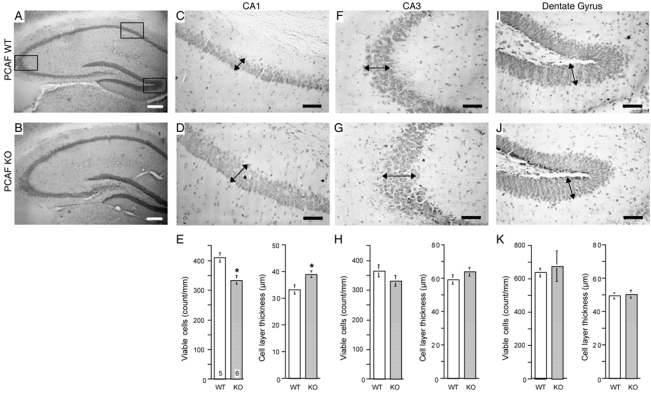
Morphological examination of the hippocampal formation of PCAF KO mice. Typical photomicrographs of the whole hippocampus (A, B), CA1 pyramidal neuronal layer (C, D), CA3 layer (F, G) and the dentate gyrus (I, J) of WT (A, C, F, I) and PCAF KO (B, D, G, J) mice. E, H, K, Quantification of the layer thickness in the three hippocampal areas. Squares in A show the localization of CA1, CA3 and the dentate gyrus. Arrows in C—J point out where the thickness was measured using NIH software. Quantification was based on the mean value for 3–6 slices per animal and the number of animals indicated within columns in (E). Scale bar = 100 μm in A, B; 50 μm in C—J. * p < 0.05 vs. the WT value (Student’s t-test).
Synaptic plasticity in PCAF KO mice
The behavioral data suggested perturbations in normal synaptic function or synaptic plasticity as a basis for the short-term memory deficits in PCAF KO mice. We therefore examined two physiologic responses in PCAF KO animals subjected to different behavioral paradigms. First, c-fos induction in basal conditions and after completion of the object recognition task was examined in the hippocampal formation by Fos protein immunohistochemistry (Fig. 5). Secondly, the induction of MAP kinases, measured through phosphorylation of ERK1/2, was examined in WT and PCAF KO mice after the passive avoidance procedure (Fig. 6).
Figure 5.
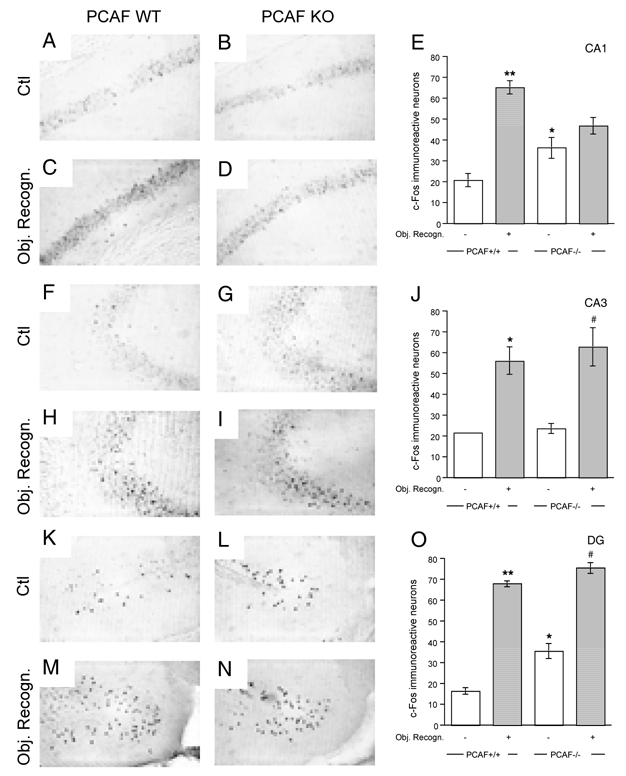
Alterations in hippocampal activation, measured through Fos expression in basal conditions and after an object recognition task in PCAF KO mice. WT or PCAF KO male mice were used in control conditions (Ctl) or after behavioral examination in the object recognition procedure (Obj. Recogn.). The latter animals were sacrificed 1 h after the task. The left panel shows typical photomicrographs of Fos immunoreactivity in the CA1 (A—D), CA3 (F—I) and dendate gyrus (DG) areas (K—N) of the hippocampus in wild-type and PCAF KO mouse brains. E, J, O, The right panel shows average Fos immunoreactivity density measurements. Scale bars = 100 μm. * p < 0.05 vs. the Ctl wild-type mouse value, # p < 0.05 vs. the Ctl PCAF KO mouse value (Newman-Keuls’ test).
Figure 6.
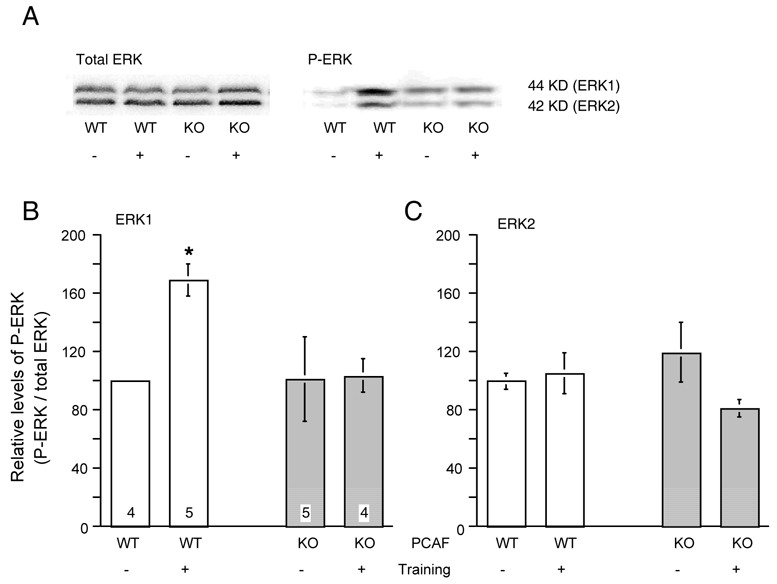
Alterations in hippocampal activation, measured through phosphorylation of ERKs in basal conditions and after passive avoidance training, in PCAF KO mice. (A) Representative picture showing the effect of training in WT and PCAF KO mice on phosphorylated ERK1 and ERK2 protein expression (−: control animals; +: trained animals). (B, C) Ratios of phosphorylated ERK (P-ERK) to total ERK in the hippocampus, expressed as a percentage of the control WT mice value. Animals were trained in the passive avoidance test and retention was performed after 24 h. Trained animals and naive controls were immediately sacrificed and hippocampi were used for Western blot analyses. The number of animals per group is indicated within columns in (B). Data were analyzed using a Kruskal-Wallis ANOVA. * p < 0.05 vs. control WT mice value (Dunn’s test).
The Fos-immunopositive cells were measured in the hippocampal CA1 (Fig. 5A–5E), CA3 (Fig. 5F–5J) and dendate gyrus areas (Fig. 5K–5O). They were analyzed using two-way ANOVA with behavior and substrain as independent factors. In CA1, a significant effect was measured for behavior (F(1,8) = 12.48, p < 0.01) and the interaction (F(1,8) = 6.56, p < 0.05; Fig. 5E). In CA3, a significant effect was measured only for behavior (F(1,8) = 9.85, p < 0.05; Fig. 5J). In the dendate gyrus, significant effects were observed for both behavior (F(1,8) = 80.0, p < 0.0001) and substrain (F(1,8) = 7.04, p < 0.05; Fig. 5O), but not for the behavior × substrain interaction. In wild-type animals, the basal level of Fos-immunoreactive neurons was low and similar among areas. The behavioral examination led to a significant increase in Fos-immunoreactivity in each area. In PCAF KO mice, the basal level of Fos-immunoreactive neurons was significantly higher than controls in CA1 and in the dentate gyrus and the behavioral-induced increase was observed only in the CA3 and dentate gyrus areas (Fig. 5). Alteration of hippocampal neuronal activation was thus observed in PCAF KO mice.
The level of ERK1/2 phosphorylation was measured in the hippocampus of WT and PCAF KO mice. Two batches of animals were assayed, i.e. mice submitted to the passive avoidance procedure and naive, behaviorally non-trained, animals. Trained animals showed similar passive avoidance performances (step-down latency: 151 ± 61 s, n = 5, for WT mice vs. 190 ± 45 s, n = 4, for WT mice). They were sacrificed immediately after the retention session. As shown in Fig. 6B, the behavioral testing induced a significant increase in ERK1 phosphorylation in WT, but not in PCAF KO mice. The behavioral procedure did not significantly affect the level of ERK2 phosphorylation in both WT and PCAF KO mice (Fig. 6C).
Emotional state and response to stress of PCAF KO mice
The response to acute stress of PCAF KO mice was first tested in the forced swimming stress test (Fig. 7). During the initial session, PCAF KO showed an increased immobility duration over the 6-min trial. Two-way ANOVA analyses showed significant effects for the time (F(5,150) = 51.1, p < 0.0001), substrain (F(1,150) = 64.8, p < 0.0001) and the time × substrain interaction (F(5,150) = 5.58, p < 0.0001). Indeed, after the second minute, the immobility duration shown by PCAF KO was significantly higher than that of WT control animals (Fig. 7A). The analysis of the immobility duration, minute per minute, showed that PCAF KO mice remained immobile an average of 40 s/min after the second minute, i.e. significantly more than WT, about 20 s/min (ANOVA: F(5,150) = 8.83, p < 0.0001 for the time, F(1,150) = 54.1, p < 0.0001 for the substrain, F(5,150) = 1.28, p > 0.05 for the time × substrain interaction, Fig. 7B). During the second day session, mice developed a marked response to the learned stressful situation by showing increased immobility duration. However, PCAF KO still showed an increased response as compared with WT (Fig. 7C). Two-way ANOVA analyses showed significant effects for the time (F(5,150) = 89.8, p < 0.0001), substrain (F(1,150) = 13.3, p < 0.001) but not for the time × substrain interaction (F(5,150) = 0.66, p > 0.05). Indeed, the immobility duration shown by PCAF KO was not significantly different from that of WT (Fig. 7C). The analysis of the immobility duration minute per minute showed that both PCAF KO and WT mice tended to remain immobile an average of 40 s/min and a significant difference between PCAF KO and WT animals was only measured during the 4th min (ANOVA: F(5,150) = 3.26, p < 0.01 for the time, F(1,150) = 8.10, p < 0.01 for the substrain, F(5,150) = 0.10, p > 0.05 for the time × substrain interaction, Fig. 7D). The cumulative immobility duration shown by the mice during the last 5 min, which is classically used to screen for antidepressant-like activity, confirmed that PCAF KO mice remained, on day 1, significantly more immobile than WT controls and showed that the level of immobility was comparable to the level reached by WT on day 2 (Fig. 7E). Moreover, PCAF KO failed to show an augmentation in the global immobility score between days 1 and 2.
Figure 7.
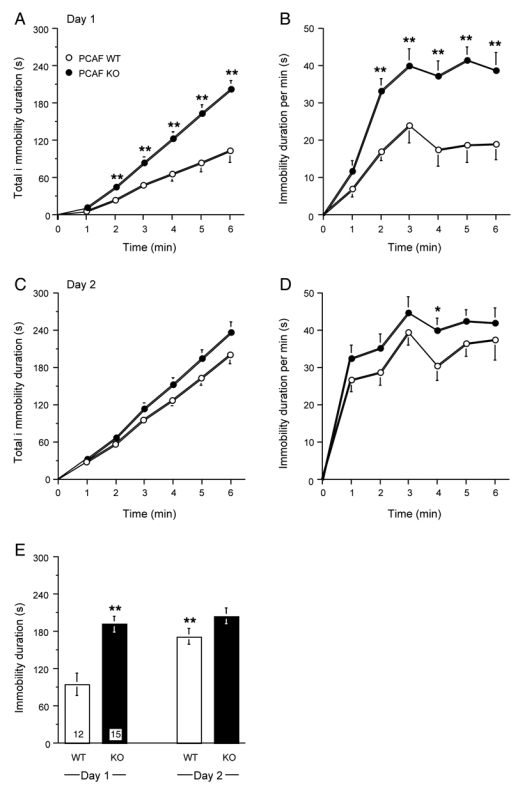
Increased behavioral despair response in PCAF KO mice in the forced swimming test. Responses of 2 month old WT and PCAF KO mice in the forced swimming test: (A, B) time-course of the immobility duration and distribution of the immobility minute per minute, during the first 6 min of the session on day 1; (C, D) time-course of the immobility duration and distribution of the immobility minute per minute, during the 6 min of the session on day 2; (E) global immobility score between min 1—6. The number of animals per group is indicated within columns in (E). In (A—D), data were analyzed using a two-way ANOVA with substrain and time as independent factors. ** p < 0.01 vs. WT value for the same time. In (E), data were analyzed using a two-way ANOVA with substrain and day as independent factors. ** p < 0.01 vs. WT value on day 1 (Newman-Keuls’ test).
Animals were then tested in the conditioned fear stress test (Fig. 8). This test includes anxiety impact in the response to stress, since animals are re-tested in a stressor-free situation. As shown in Fig. 8A, non-shocked PCAF KO mice exhibited a significantly lower ambulatory activity in the arena, as shown over the first 6 min of the session on day 1 (ANOVA: F(5,132) = 24.9, p < 0.0001 for the time, F(1,132) = 66.0, p < 0.0001 for the substrain, F(5,132) = 1.24, p > 0.05 for the time × substrain interaction, Fig. 8A). Both WT and PCAF KO mice exhibited lower ambulatory activity when submitted to footshocks (Fig. 8B), but PCAF KO mice still exhibited significantly lower activity (F(5,180) = 63.2, p < 0.0001 for the time, F(1,180) = 77.0, p < 0.0001 for the substrain, F(5,180) = 1.21, p > 0.05 for the time × substrain interaction, Fig. 8B). Indeed, significant differences were measured at all measure timepoints. No difference among groups was measured on the test day for non-shocked animals (F(5,132) = 17.3, p < 0.0001 for the time, F(1,132) = 1.54, p > 0.05 for the substrain, F(5,132) = 0.05, p > 0.05 for the time × substrain interaction, Fig. 8C). However, shocked PCAF KO showed very low ambulatory activity, which was significantly lower than the response of control WT mice (F(5,180) = 8.36, p < 0.0001 for the time, F(1,180) = 63.6, p < 0.0001 for the substrain, F(5,180) = 0.42, p > 0.05 for the time × substrain interaction, Fig. 8D). Global activity scores are presented in Figs. 7E, F. Non-shocked PCAF KO mice showed significantly lower activity on day 1, similar to that observed in WT or themselves on day 2 (F(1,44) = 6.01, p < 0.05 for the substrain, F(1,44) = 7.15, p < 0.05 for the day, F(1,44) = 5.37, p < 0.05 for the substrain × day interaction, Fig. 8E). This observation, which reflected an anxious reactivity to novelty, is in line with the increased object exploration measured during the first sessions in the object recognition test. In shocked animals, PCAF KO showed a lower activity than WT animals on day 1 during the shock application, but a significant stress-induced decrease in ambulatory activity was still observed between days 1 and 2 (F(1,60) = 20.6, p < 0.0001 for the substrain, F(1,60) = 121.8, p < 0.0001 for the day, F(1,60) = 0.86, p > 0.05 for the substrain × day interaction, Fig. 8F). In particular, on day 2, PCAF KO mice showed significantly lower activity as compared with WT mice.
Figure 8.
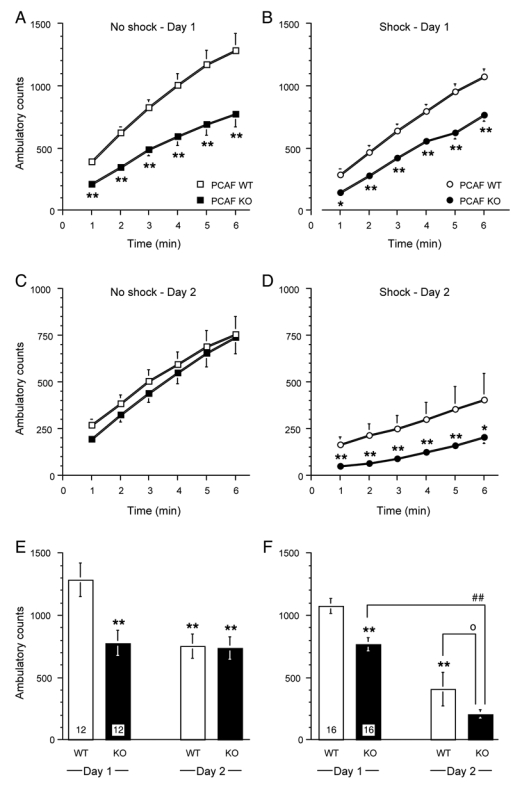
Increased freezing response in PCAF KO mice in the conditioned fear stress test. Responses of 2 month old WT and PCAF KO mice in the conditioned fear stress test: Time-course of ambulatory activity during the first 6 min of the session on day 1 for non-shocked control animals in (A) and shocked animals in (B). Time-course of the ambulatory activity during 6 min of the session on day 2 for non-shocked control animals in (C) and shocked animals in (D). Global activity score over 6 min for non-shocked control animals in E and shocked animals in F. The number of animals per group is indicated within columns in (E, F). In (A—D), data were analyzed using a two-way ANOVA with substrain and time as independent factors. * p < 0.05, ** p < 0.01 vs. WT value for the same time. In (E, F), data were analyzed using a two-way ANOVA with substrain and day as independent factors. ** p < 0.01 vs. WT value on day 1, ## p < 0.05 vs. day 1 value, o p < 0.05 vs. WT value on the same day (Newman-Keuls’ test).
These results showed that PCAF KO mice presented a marked hyper-responsivenness to acute stress. Hyper-activation of the hypothalamo-pituitary-adrenal axis results notably in an augmentation of corticosterone from the adrenals. Plasma corticosterone levels were therefore measured in control WT and PCAF KO mice and in animals submitted to 15 min forced swimming. As shown in Fig. 9, non-stressed PCAF KO mice presented a highly significant +200% increase in corticosterone contents. The 15-min forced swimming stress significantly increased corticosterone levels in WT and also in KO mice, that remained able to respond to the stress. However, PCAF KO mice presented similar corticosterone levels as stressed WT animals (Fig. 9).
Figure 9.
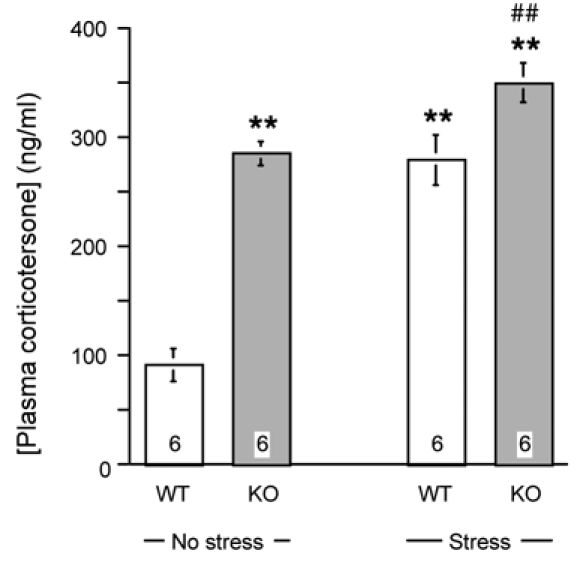
Plasma corticosterone levels in PCAF KO mice. Blood samples were taken from WT or PCAF KO mice immediately after 15 min forced swimming stress or from non-stressed control animals. The number of animals per group is indicated within columns. ** p < 0.01 vs. non-stressed WT value; ## p < vs. stressed WT value (Newmann-Keuls’ test).
Constitutively high corticosterone levels may lead to a chronic depressive state. Therefore, a putative anhedonic state, likely to be associated with depression, was investigated. The spontaneous drinking preference for a sucrose solution was measured over 9 days. As shown in Fig. 10A, the tap water and sucrose intake patterns did not differ among substrains (tap water: F(8,81) = 3.41, p < 0.01 for the day, F(1,81) = 0.26, p > 0.05 for the substrain, F(8,81) = 0.41, p > 0.05 for the day × substrain interaction, sucrose: F(8,81) = 4.18, p < 0.001 for the day, F(1,81) = 0.15, p > 0.05 for the substrain, F(8,81) = 0.64, p > 0.05 for the day × substrain interaction Fig. 10A). Moreover, both WT and PCAF KO animals gradually developed a high (about 80%) sucrose preference after a couple of days (F(8,81) = 4.75, p < 0.01 for the day, F(1,81) = 0.68, p > 0.05 for the substrain, F(8,81) = 0.74, p > 0.05 for the day × substrain interaction, Fig. 10B). The PCAF KO mice could therefore not be considered as spontaneously depressed.
Figure 10.
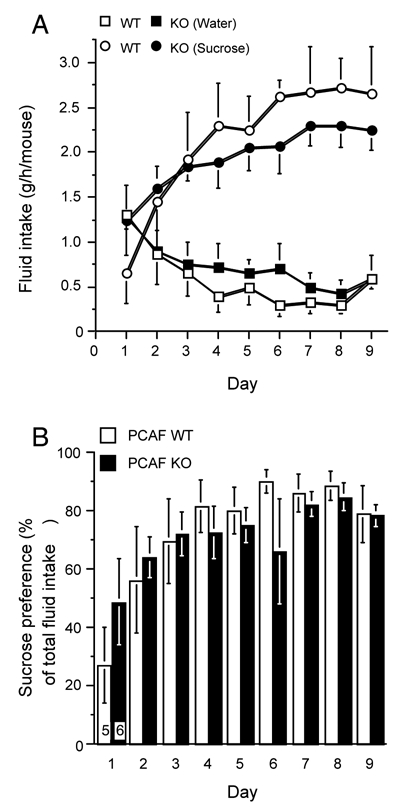
Preserved haedonic response in PCAF KO mice. (A) Fluid consumption profiles (water: squares; sucrose 4%: circles) for 2 month old WT (open symbols) and PCAF KO mice (filled symbols); (B) Sucrose preference expressed as a percentage of total fluid intake in the sucrose preference test. Animals were submitted to water restriction 16 h before being presented one bottle of water and one bottle of 4% sucrose solution. Fluid consumption was measured after 1 h. The data were analyzed using a two-way ANOVA with substrain and day as independent factors. No statistical difference was measured.
Age-related memory impairment patterns in PCAF KO mice
Male mice were tested again at 6 months of age and female mice at 12 months of age, in order to determine whether the memory impairment would evolve with age. We examined these middle-aged animals in the open-field, water-maze learning and passive avoidance tests.
At 6 months of age, PCAF KO animals showed significant increases in the latency to leave the center ring of the arena, and in the numbers of rearing and grooming reactions (Table 2). In addition, a non-significant trend towards increased total locomotion, locomotion at the center of the arena and exploratory speed was noted (Table 2). At 12 months of age, PCAF KO mice still showed a significantly increased number of rearing reactions and a significant decrease in the locomotion at the center of the arena (Table 2). A non-significant trend towards increased total locomotion and exploratory speed were still observed (Table 2). It was thus concluded that PCAF KO mice developed some perturbations in exploration behavior and stereotyped behaviors with age, which may contribute to the expression of cognitive deficits.
Table 2.
Behavioral parameters of 6 and 12 month old PCAF KO and WT mice in the open-field test
| Parameter | 6-m.o. male mice
|
12-m.o. female mice
|
||
|---|---|---|---|---|
| WT | PCAF KO | WT | PCAF KO | |
| Weight (g) | 36.0 ± 0.6 | 35.5 ± 1.2 | 32.2 ± 0.9 | 36.3 ± 1.0 |
| Latency to start (s) | 1 ± 0 | 9 ± 2 * | 0 ± 0 | 1 ± 0 |
| Locomotion (m) | 27.6 ± 2.4 | 35.8 ± 1.9 | 14.6 ± 2.2 | 20.3 ± 3.3 |
| Loc. in center (%) | 23.4 ± 1.9 | 31.1 ± 2.0 | 29.8 ± 4.2 | 17.4 ± 2.0 * |
| Speed (m/min) | 3.29 ± 0.20 | 4.28 ± 0.19 | 2.60 ± 0.18 | 3.30 ± 0.33 |
| Immobility (s) | 98 ± 25 | 93 ± 7 | 285 ± 36 | 243 ± 31 |
| Rearing | 14 ± 2 | 52 ± 7 ** | 8 ± 3 | 20 ± 2 ** |
| Grooming | 5 ± 0 | 24 ± 2 ** | 5 ± 1 | 5 ± 1 |
| Defecations | 2 ± 0 | 3 ± 0 | 4 ± 1 | 6 ± 0 |
| n | 8 | 4 | 12 | 13 |
p < 0.05,
p < 0.01 vs. the age-matched WT group, Student’s t-test.
In the water-maze test, both 6 month old WT and PCAF KO mice showed decreased latencies in finding the platform during acquisition of the fixed platform location (Fig. 11A). The statistical analysis showed significantly lower latencies for both wild-type (F(6,55) = 18.66, p < 0.0001) and PCAF KO mice (F(6,27) = 11.90, p < 0.001; Fig. 11A). However, latencies shown by PCAF KO animals were augmented as compared with wild-type mice between trials 2 and 6 (Fig. 11A). However, during the probe test, only wild-type animals preferentially swam in the T quadrant (F(3,27) = 5.54, p < 0.01; Fig. 10B) and not PCAF KO (F(3,15) = 0.24, p > 0.05), indicating that PCAF KO mice developed long-term memory deficits. WT mice showed a classical profile for the acquisition of a daily changing platform location (Fig. 11C). A substantial decrease between the 1st and 2nd trial latency was measured. PCAF KO animals showed impaired short-term memory, ascribed to a significant increase in the trial 2 latency (p < 0.05; Fig. 11C). Note that the deficit in long-term memory observed in this spatial procedure in the water-maze test was not reproduced in a contextual procedure, i.e. a passive avoidance test (Figs. 11D, 11E). Neither the step-down latency (Fig. 10E) nor the percentage of animals to criterion (Fig. 11E) showed a statistically significant difference between wild-type and PCAF KO animals.
Figure 11.
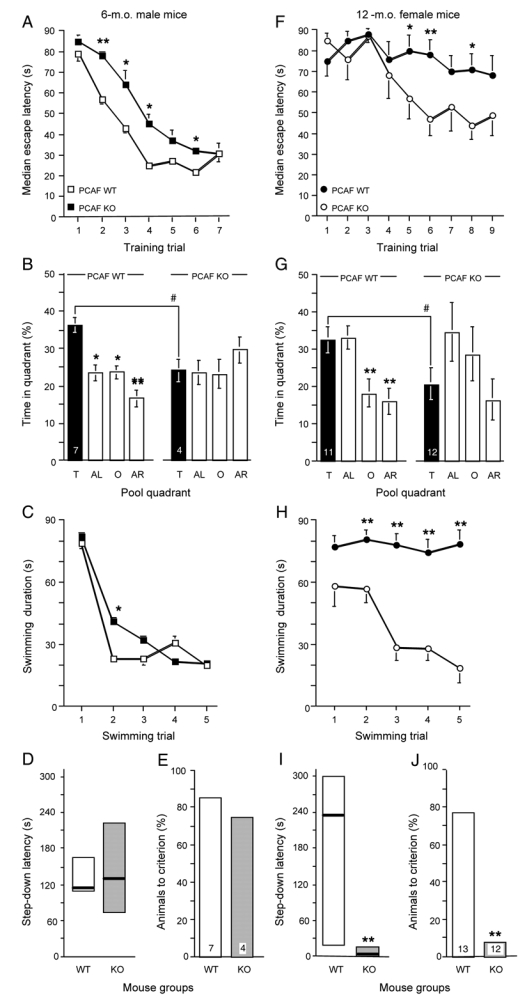
Age-related increased memory impairment in PCAF KO mice: place learning in the water-maze (A—C, F—H), and passive avoidance performance (D, E, I, J) for 6-m.o. male (A—E) and 12-m.o. female (F—J). A, F, Acquisition latencies for a fixed platform position in the water-maze test. Animals were submitted for 7 or 9 days to three swims per day, with an intertrial time interval of 10 min. * p < 0.05, ** p < 0.01 vs. the control value during the same training trial (Dunnett’s test). (B, G) One hour after the last swimming trial, the platform was removed and animals were submitted to a 60 s probe test. The presence in each quadrant was measured. Quadrants: T, training; AL, adjacent left; O, opposite; AR, adjacent right. The number of animals per group is indicated in the T column. * p < 0.05 vs. the time spent in the T quadrant (Dunnett’s test). (C, H) Short-term memory deficits were measured using the daily changing platform position procedure: swimming latency profiles among the 5 trials, * p < 0.05, ** p < 0.01 vs. the control value (Dunn’s test). Passive avoidance behavior: (D, I) step-down latency; (E, J) percentage of animals to criterion. The number of animals is indicated in (E, J). ** p < 0.01 vs WT data (Mann-Whitney’s test in (I),χ2 test in (J)).
The 12-m.o. PCAF KO animals presented higher impairment levels. In the water-maze test, only WT, but not PCAF KO, mice showed lower latencies (F(8,98) = 29,69, p < 0.001 for WT; F(8,98) = 9.18, p > 0.05 for PCAF KO; Fig. 11F). Moreover, latencies shown by PCAF KO animals appeared to be significantly higher than that observed for wild-type mice between trials 5, 6 and 8 (Fig. 11F). During the probe test, only WT animals preferentially swam in the T quadrant (F(3,44) = 8.61, p < 0.0001; Fig. 11G), contrary to PCAF KO (F(3,44) = 1.85, p > 0.05). Moreover, the difference in time spent in the T quadrant between each group was significant. In the short-term memory procedure, WT mice acquired the daily changing platform location (Fig. 11H), since a marked decrease in latencies was measured (F(4,49) = 2.83, p < 0.05; Fig. 11H). PCAF KO animals showed impaired short-term memory, since no significant decrease in latency was observed over the swimming trials (F(4,59) = 0.40, p > 0.05; Fig. 11H). The latencies measured for trials 2 to 5 were significantly higher as compared with WT animals (Fig. 11H). Passive avoidance deficits were measured for 12-m.o. PCAF KO animals, both in terms of step-down latency (Fig. 11I) and percentage of animals to criterion (Fig. 11J), indicating that contextual long-term memory was also affected at this age.
DISCUSSION
The p300/CBP associated factor (PCAF) histone acetylase shares a double function, acting as a transcriptional coactivator and as a histone acetyltransferase, and contributing to transcriptional activation during gene transcription by allowing nucleosome acetylation and chromatin structure remodeling (Ogryzko et al, 1996; Yamauchi et al, 2000; Korzus et al, 2004). The targeted inactivation of PCAF in CD1 mice failed to affect the level of histone H3 acetylation, suggesting that other(s) histone acetyltransferase(s) mediated H3 acetylation on Lys14. In particular, GCN5 also target Lys14 on histone H3. However, it can not be excluded that histone H3 acetylation on specific genes may be impaired in PCAF KO mice, resulting in a complex phenotypic pattern. PCAF KO animals were viable, reached sexual maturity and could be bred normally, contrary to GCN5 or CBP homozygous KO animals (Yamauchi et al, 2000). However, behavioral and physiological examination showed marked learning and memory impairments and an exagerated response to acute stress. Moreover, contrary to heterozygous CBP KO models, PCAF KO mice initially showed impairments in short-term memory that affected long-term memory only later in life.
Behavioral phenotyping of 2-m.o. PCAF KO mice using tests to assess general activity and memory functions initially showed that the general activity of PCAF KO mice resembled that of wild-type controls. Open-field examination, in particular, showed similar measures for parameters related to locomotion, exploration, emotional response and stereotyped behaviors. Secondly, no sex-related difference was noted regardless of the behavioral parameter examined.
However, we observed marked alterations in the short-term memory component, shown by deficits in spontaneous alternation, significant delay in escape latencies during water-maze acquisition sessions and significant increases in swimming latencies during the procedure involving a daily changing platform position (Meunier and Maurice, 2004). The delay in acquisition of the platform position was related to short-term memory impairment. Indeed, repetitive training of a fixed platform position involved short-term memory among trials within the same training day, and reference memory among training days. Global impairment of both short-term and reference spatial memory components will lead to learning impairments that were observed during the probe test. Impairment of the short-term or reference memory components may induce a significant delay in the acquisition profile due to worsening of the learning quality during each training trial that, however, could be attenuated through repetition of learning trials without any obvious probe test deficit. Recognition memory, another form of short-term memory, was markedly impaired in PCAF KO mice. When placed in the presence of four different objects, PCAF KO mice showed an increased level of interaction with objects that altered the quality of habituation and blocked the correct reaction to object position changes. Perturbations in reaction to novelty, exploration or short-term memory may be involved in the observed pattern. Interestingly, during session 7, PCAF KO mice displayed a correct reaction to the presence of the novel object, ultimately suggesting that animals become familiar with the three other objects but also that mnesic processes selectively involving the hippocampal formation, such as change in object position, were more altered than less hippocampus-dependent processes, such as the discrimmination between novel and familiar objects (Ennaceur and Delacour, 1988). Finally, short-term memory impairment could likely explain the deficits in object recognition observed in PCAF KO mice.
Long-term memory appeared, however, to be preserved in young PCAF KO mice. Animals could acquire the fixed position of the platform in the water-maze and showed correct passive avoidance learning performances. The key finding of our study is thus that the memory impairment profile shown by PCAF KO mice differs from previously reported patterns in heterozygous CBP KO and CBP{HAT−} animals. CBP KO mice showed long-term memory deficits, measured using passive avoidance, fear conditioning and a long-term object recognition procedure (Oike et al, 1999; Alarcon et al, 2004). Short-term memory, measured using spontaneous alternation or a short-term memory version of the radial-arm maze task, was mainly preserved. However, Oike et al (1999) reported hypolocomotor activity in CBP KO mice, whereas Alarcon et al (2004) observed unaffected open-field parameters, anxiety responses, sensorimotor gating and motor coordination responses. These differences could be attributed not only to the different inactivation strategies, but also to the different genetic backgrounds of the transgenic animals, C57BL/6 or C57BL/6 × BALB/C hybrids (Oike et al, 1999; Alarcon et al, 2004). The selective alteration of long-term memory processes together with preservation of short-term memory in CBP KO mice suggested that memory consolidation, storage and/or long-term retrieval, but not acquisition, are selectively affected, and that these animals may represent a model for the cognitive deficits in Rubinstein-Taybi syndrome (Oike et al, 1999; Alarcon et al, 2004). Mental retardation in Rubinstein-Taybi syndrome patients is characterized by low intelligence, a short attention span, and major difficulties in planning and executing motor skills (Gotts and Liemohn, 1977). Its modeling in animal tests could be assessed through general hypoactivity and learning impairment consistent with the deficits observed in CBP KO mice.
The differences observed in the behavioral phenotypes of CBP KO and PCAF KO animals suggest that the embryonic inactivation of PCAF, and the resulting alteration of gene expressions, directly affect the synaptic plasticity and rapid neuronal signaling involved in short-term memory. The hippocampus is critically involved in different forms of memory, including spatial and recognition memory. Morphological examination of the pyramidal cell layer morphology revealed significant cell loss associated with thickening of the CA1 area in PCAF KO mice. Changes in the CA3 area were much less marked and not significant. A more extensive morphofunctional study using specific markers is needed. However, this observation suggests that postnatal developmental alterations in PCAF KO mice have morphological consequences. We assessed two hippocampal-dependent physiological responses. First, Fos expression was measured in basal conditions and after activation by the object recognition procedure in wild-type and PCAF KO animals. Basal Fos expression increased in CA1 and the dendate gyrus in PCAF KO mice. Moreover, the increase in Fos expression induced by the behavioral procedure was significantly attenuated in the CA1 area, denoting a clear hyporesponsiveness of CA1 neurons. These observations were likely not related to morphological alterations in the cell layers, since the extent of the increase in basal Fos expression (+74%) was higher than the differences in cell density (−18%). Moreover, this pattern was also observed in the dentate gyrus. Secondly, we analyzed the induction of MAP kinase activity by measuring phophorylated ERK levels. PCAF KO mice showed similar levels of phospho-ERK in basal conditions, whereas training associated induction of phospho-ERK expression was observed in WT mice but not in PCAF KO mice. Animals underwent passive avoidance training, i.e. a procedure known to induce rapid and sustained ERK activation (Han and Kim, 2003; Lee et al, 2004). However, ERK induction, and particularly ERK1 phophorylation, may not be a critical prerequisite for a preserved passive avoidance response (Selcher et al, 2001). Therefore, these experimental conditions were ideal because they highlighted that the physiological plasticity involved in learning was impaired in PCAF KO animals although they showed no major deficit in behavioral response. Both modified responses in PCAF KO mice suggest that the absence of histone acetylation results in a complex pattern of deficits resulting from modification of gene expression levels under basal conditions or stimulated conditions.
PCAF KO mice were retested at the behavioral level at older ages. Since mice were housed in groups and presented a high level of endogenous corticosterone, we were not surprised to find, partricularly in male animals, a high tendency to fight with each other and to show a rapid progressive degradation in their general features. Males were therefore selected to be tested at 6 months of age and females at 12 months of age. Adult, but not senescent or even aged, animals were therefore considered. Short-term memory impairments were still observed in water-maze acquisition profiles and in the daily changing platform position procedure. However, long-term memory deficits were noteworthy, since the probe test performances were very poor for PCAF KO mice at both ages. Moreover, passive avoidance performances, which were unaltered at 6-m.o., showed marked deficits at 12-m.o., indicating that contextual long-term memory was affected. Therefore, memory impairments observed in PCAF KO mice changed with age towards long-term memory deficits affecting all components of the mnesic process, although emotionally reinforced memory appeared to be preserved longer.
Using a model of chronic social defeat, Tsankova et al (2006) recently showed that stress downregulated Bdnf transcripts III and IV and long-lastingly increased histone H3 methylation at their respective promoters, therefore repressing gene transcription. Moreover, an antidepressant treatment with imipramine reversed this downregulation and resulted in increased acetylation of H3 histone at the promoters (Tsankova et al, 2006). These important results suggested a direct role of the chromatin state, with transcription being repressed through histone hypermethylation or activated through histone hyperacetylation, in the molecular effectors sustaining physiological allostasis to acute stress. Here we report that PCAF KO mice presented an increased response to acute stress. This was observed when mice were submitted to the forced swimming procedure, i.e. the most commonly used method for evaluating depression-like behavior and screening for antidepressant-like activity, and to the conditioned fear stress test, which is a psychological stress procedure that is sensitive to anxiolytics. This increased sensitivity was explained by augmented basal levels of plasma corticosterone in PCAF KO mice. Moreover, since PCAF KO were still able to respond to the stress, not only in terms of behavioral responses, but also in terms of corticosterone secretion, it appears that these mice presented a modification in their allostatic state, rather than a constitutive stressed phenotype. In agreement with this conclusion, PCAF KO mice did not show marked anhedonia, suggesting that they may not be constitutively depressive. PCAF thus seems to play a role in the chromatin remodelling involved in the regulation of gene expression controlling the emotional state and physiological allostasis. Moreover, PCAF could be involved in the histone acetylation sustaining acute antistress effects of antidepressant drugs. These observations pave the way for a new avenue of research on antistress or antidepressant compounds.
Noteworthy, corticosterone has been reported to affect memory processes. Repeated administration of corticosterone facilitates the acquisition of emotional memory, particularly by improving the consolidation and retention phases, ensuring the transition from short- to long-term memory (Sandi and Rose, 1994, 1997; Coburn-Litvak et al, 2003; Thompson et al, 2004). Therefore, the high corticosterone levels measured in PCAF KO mice may interfer with long-term memory, particularly as measured in the passive avoidance procedure. Consolidation processes facilitated by the high corticosterone tonus may compensate impaired learning, and account for the preserved passive avoidance performances as compared with alternation performance deficits. This may be particularly true for 6-m.o. animals, since emotional learning appeared more preserved than spatial learning capacities.
The PCAF KO mouse thus appears to be a complex animal model exhibiting developmental and functional deficits that change during life. This study provides evidence that PCAF is involved in learning and memory processes and response to stress, but differently than CBP and related histone acetyltransferases. In particular, the PCAF KO mouse may constitute a unique model for screening anti-amnesic and anti-depressant activity, while taking the role of chromatin remodelling into account as a key mechanism controlling long-term adaptative changes associated with psychiatric conditions.
Acknowledgments
The behavioral analysis was a project (#01) of the CompAn animal behavior phenotyping facility (Montpellier, France). Thanks are due to Dr Monica Prieto (Montpellier) for technical advice in immunohistochemistry and to Dr Karim Chebli and the animal facility of the Institut de Génétique Moléculaire (IGMM, IFR122, Montpellier) for mouse breeding.
References
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Blanco JC, Minucci S, Lu J, Yang XJ, Walker KK, Chen H, Evans RM, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1988;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci. 2004;27:280–295. doi: 10.1016/j.mcn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gotts EE, Liemohn WP. Behavioral characteristics of three children with the broad thumb-hallux (Rubinstein-Taybi) syndrome. Biol Psychiatry. 1977;12:413–423. [PubMed] [Google Scholar]
- Han Y, Kim SJ. Memory enhancing actions of Asiasari radix extracts via activation of insulin receptor and extracellular signal regulated kinase (ERK) I/II in rat hippocampus. Brain Res. 2003;974:193–201. doi: 10.1016/s0006-8993(03)02580-0. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Tagawa K, Okuda T, Murata M, Oyanagi K, Arai N, Mizutani T, Kanazawa I, Wanker EE, Okazawa H. Histone deacetylase activity is retained in primary neurons expressing mutant huntingtin protein. J Neurochem. 2003;87:257–267. doi: 10.1046/j.1471-4159.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–682. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- Janknecht R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol Histopathol. 2002;17:657–668. doi: 10.14670/HH-17.657. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lachner M, O’Sullivan RJ, Jenuwein T. An epigenetic road map for histone lysine methylation. J Cell Sci. 2001;116:2117–2124. doi: 10.1242/jcs.00493. [DOI] [PubMed] [Google Scholar]
- Lee JK, Choi SS, Lee HK, Han KJ, Han EJ, Suh HW. Effects of MK-801 and CNQX on various neurotoxic responses induced by kainic acid in mice. Mol Cell. 2004;14:339–347. [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunyak VV, Burgess R, Prefontaine GG, Nelson C, Sze SH, Chenoweth J, Schwartz P, Pevzner PA, Glass C, Mandel G, Rosenfeld MG. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science. 2002;298:1747–1752. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;9:662–671. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumi A, Wang IM, Lefebvre B, Yang XJ, Nakatani Y, Ozato K. The histone acetylase PCAF is a phorbol-ester-inducible coactivator of the IRF family that confers enhanced interferon responsiveness. Mol Cell Biol. 1999;19:1810–1820. doi: 10.1128/mcb.19.3.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J, Maurice T. Beneficial effects of the sigma1 (σ1) receptor agonists igmesine and dehydroepiandrosterone against the learning impairments in rats prenatally exposed to cocaine. Neurotoxicol Teratol. 2004;26:783–797. doi: 10.1016/j.ntt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFN b expression by disrupting the enhanceosome. Mol Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Kotani T, Zhang X, Schiltz RL, Howard T, Yang XJ, Howard BH, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SP. Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance learning paradigm. Brain Res. 1994;647:106–112. doi: 10.1016/0006-8993(94)91404-4. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SP. Protein synthesis- and fucosylation-dependent mechanisms in corticosterone facilitation of long-term memory in the chick. Behav Neurosci. 1997;111:1098–1104. doi: 10.1037//0735-7044.111.5.1098. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Puri PL, Hamamori Y, Ogryzko VV, Chung G, Nakatani Y, Wang JY, Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Nekrasova T, Paylor R, Landreth GE, Sweatt JD. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn Mem. 2001;8:11–9. doi: 10.1101/lm.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M, Kurokawa R, Housman DE, Jackson GR, Marsh JL, Thompson LM. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga-Weisz P. Chromatin remodeling factors and DNA replication. Prog Mol Subcell Biol. 2005;38:1–30. doi: 10.1007/3-540-27310-7_1. [DOI] [PubMed] [Google Scholar]
- Xu L, Lavinsky RM, Dasen JS, Flynn SE, McInerney EM, Mullen TM, Heinzel T, Szeto D, Korzus E, Kurokawa R, Aggarwal AK, Rose DW, Glass CK, Rosenfeld MG. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Yamauchi J, Kuwata T, Tamura T, Yamashita T, Bae N, Westphal H, Ozato K, Nakatani Y. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc Natl Acad Sci USA. 2000;97:11303–11306. doi: 10.1073/pnas.97.21.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]


