Abstract
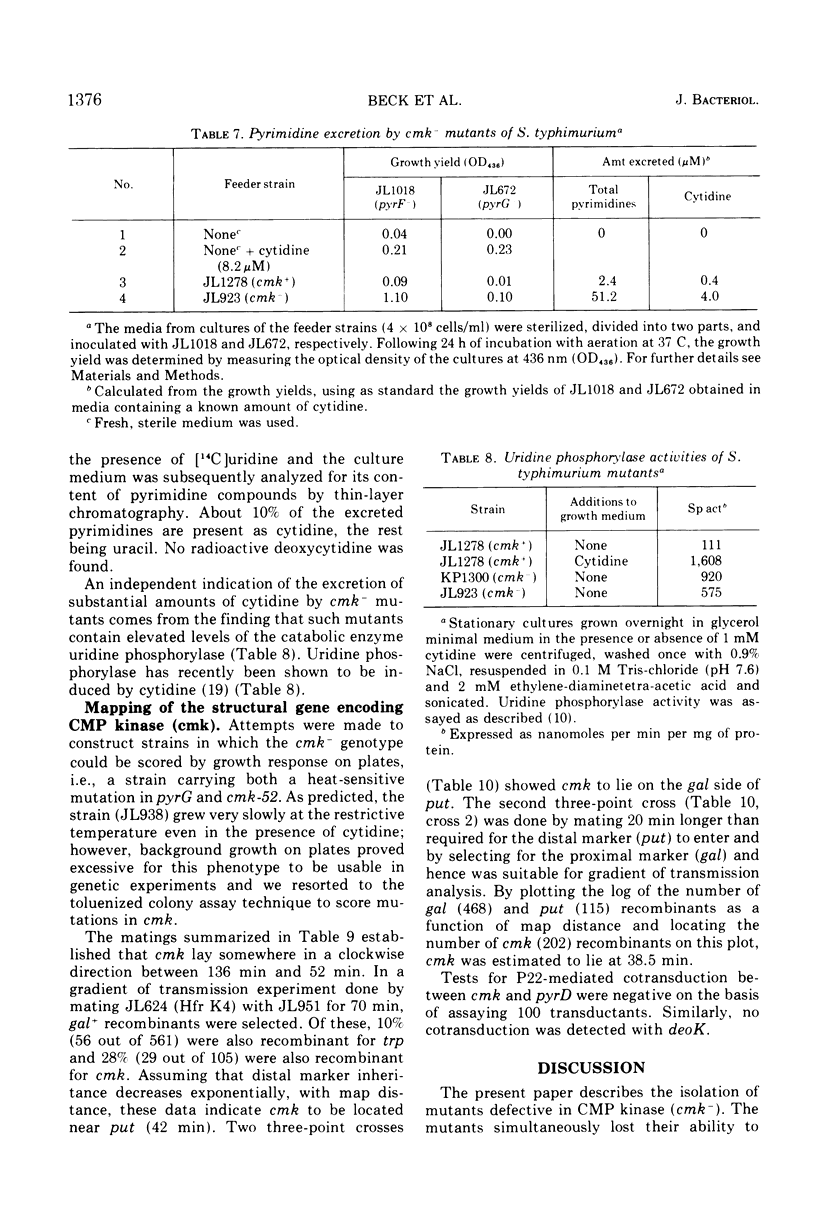
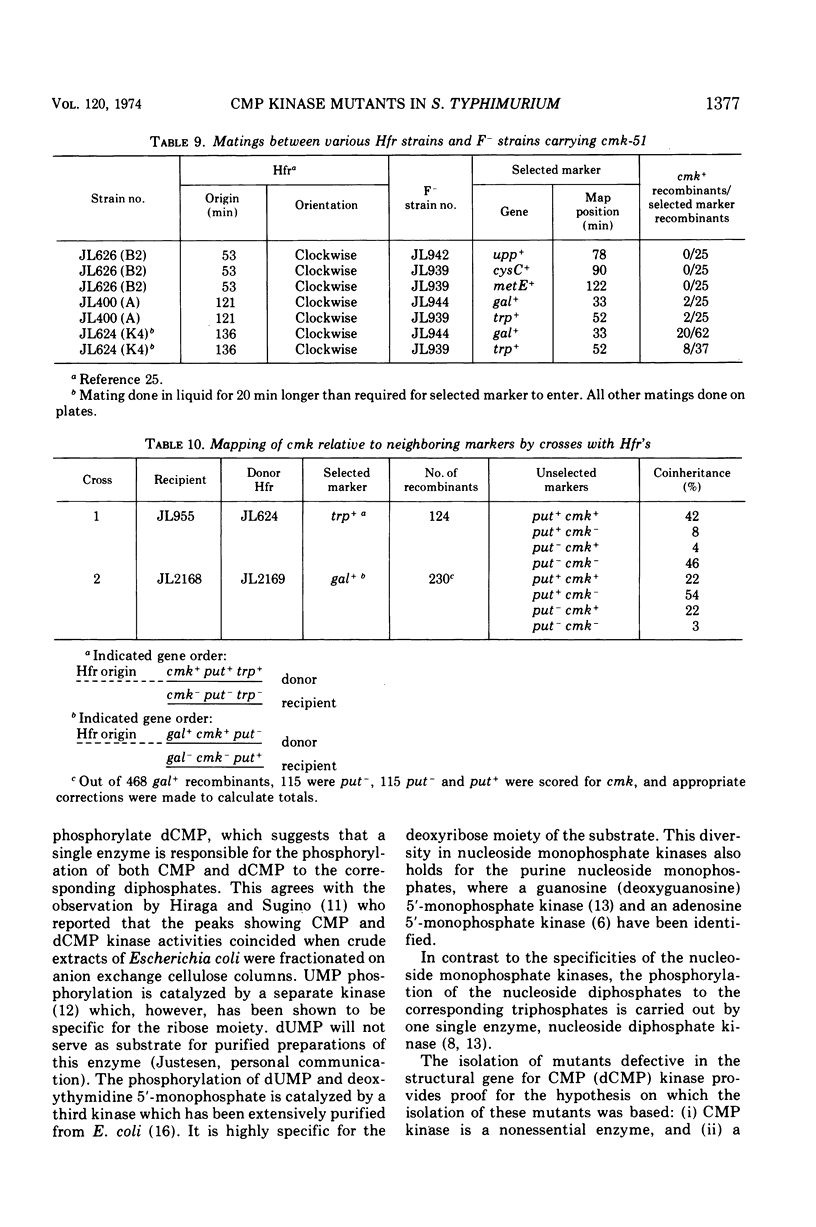
Mutants of Salmonella typhimurium defective in cytidine 5′-monophosphate (CMP) kinase (cmk) have been isolated. The mutants also lack the ability to phosphorylate 2′-deoxyCMP, indicating that one enzyme is responsible for the phosphorylation of both CMP and deoxyCMP to the corresponding diphosphates. In glucose minimal medium the mutants grow at the same rate as the parental strain; however, they excrete large quantities of pyrimidines into the growth medium. Cytidine but not deoxycytidine has been identified among the excreted products. The mutant phenotype suggests that the physiological role of CMP kinase is that of rephosphorylating CMP arising from the breakdown of messenger ribonucleic acid. This proposed role of CMP kinase is supported by the fact that a cmk− mutant is much more sensitive to any partial impairment of cytidine 5′-triphosphate synthetase than is the cmk+ parent strain. The gene cmk has been located on the Salmonella chromosome at 38.5 min. No markers which can be cotransduced with cmk by phage P22 have been found.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck C. F., Ingraham J. L. Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. Mol Gen Genet. 1971;111(4):303–316. doi: 10.1007/BF00569782. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L., Neuhard J. Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. II. Uridine kinase, cytosine deaminase and thymidine kinase. Mol Gen Genet. 1972;115(3):208–215. doi: 10.1007/BF00268884. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L., Neuhard J., Thomassen E. Metabolism of pyrimidines and pyrimidine nucleosides by Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):219–228. doi: 10.1128/jb.110.1.219-228.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney S. G., Boyer P. D. Incorporation of water oxygens into intracellular nucleotides and RNA. II. Predominantly hydrolytic RNA turnover in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):581–591. doi: 10.1016/0022-2836(72)90084-8. [DOI] [PubMed] [Google Scholar]
- Cousin D., Buttin G. Mutants thermosensibles d'Escherichia coli K12. 3. Une mutation létale d'E. coli affectant l'activité de l'adénylate-kinase. Ann Inst Pasteur (Paris) 1969 Nov;117(5):612–630. [PubMed] [Google Scholar]
- Edlin G., Maaloe O. Synthesis and breakdown of messenger RNA without protein synthesis. J Mol Biol. 1966 Feb;15(2):428–434. doi: 10.1016/s0022-2836(66)80118-3. [DOI] [PubMed] [Google Scholar]
- Ginther C. L., Ingraham J. L. Cold-sensitive mutant of Salmonella typhimurium defective in nucleosidediphosphokinase. J Bacteriol. 1974 Jun;118(3):1020–1026. doi: 10.1128/jb.118.3.1020-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A. Mutants of Escherichia coli unable to metabolize cytidine: isolation and characterization. Mol Gen Genet. 1973 Nov 2;126(2):177–186. doi: 10.1007/BF00330992. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Sugino Y. Nucleoside monophosphokinases of Escherichia coli infected and uninfected with an RNA phage. Biochim Biophys Acta. 1966 Feb 21;114(2):416–418. doi: 10.1016/0005-2787(66)90324-8. [DOI] [PubMed] [Google Scholar]
- Ingraham J. L., Neuhard J. Cold-sensitive mutants of Salmonella typhimurium defective in uridine monophosphate kinase (pyrH). J Biol Chem. 1972 Oct 10;247(19):6259–6265. [PubMed] [Google Scholar]
- Koerner J. F. Enzymes of nucleic acid metabolism. Annu Rev Biochem. 1970;39:291–322. doi: 10.1146/annurev.bi.39.070170.001451. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Long C. W., Pardee A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem. 1967 Oct 25;242(20):4715–4721. [PubMed] [Google Scholar]
- Nelson D. J., Carter C. E. Purification and characterization of Thymidine 5-monophosphate kinase from Escherichia coli B. J Biol Chem. 1969 Oct 10;244(19):5254–5262. [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Deoxycytidine triphosphate deaminase: identification and function in Salmonella typhimurium. J Bacteriol. 1971 Feb;105(2):657–665. doi: 10.1128/jb.105.2.657-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Turnover of the deoxyribonucleoside triphosphates in Escherichia coli 15 T during thymine starvation. Eur J Biochem. 1971 May 11;20(1):36–43. doi: 10.1111/j.1432-1033.1971.tb01359.x. [DOI] [PubMed] [Google Scholar]
- Nygaard P. Nucleoside-catabolizing enzymes in Salmonella typhimurium. Introduction by ribonucleosides. Eur J Biochem. 1973 Jul 2;36(1):267–272. doi: 10.1111/j.1432-1033.1973.tb02909.x. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. Function of cytidine diphosphate-diglyceride and deoxycytidine diphosphate-diglyceride in the biogenesis of membrane lipids in Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):1098–1105. [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XIV. Separation of nucleotide sugars and nucleoside monophosphates on PEI-cellulose. Anal Biochem. 1965 Dec;13(3):575–579. doi: 10.1016/0003-2697(65)90356-8. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton M., Edlin G. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):583–588. doi: 10.1016/s0006-291x(72)80179-7. [DOI] [PubMed] [Google Scholar]


