Abstract
fMRI has become the method of choice for mapping brain activity in human subjects and detects changes in regional blood oxygenation and volume associated with local changes in neuronal activity. While imaging based on blood oxygenation level dependent (BOLD) contrast has good spatial resolution and sensitivity, the hemodynamic signal develops relatively slowly and is only indirectly related to neuronal activity. An alternative approach termed magnetic source MRI (msMRI) is based on the premise that neural activity may be mapped by MRI with greater temporal resolution by detecting the local magnetic field perturbations associated with local neuronal electric currents. We used a hybrid ms/BOLD MRI method to investigate whether msMRI could detect signal changes that occur simultaneously at the time of the production of well defined event related potentials, the P300 and N170, in regions that previously have been identified as generators of these electrical signals. Robust BOLD activations occurred after some seconds, but we were unable to detect any significant changes in the T2*-weighted signal in these locations that correlated temporally with the timings of the ERPs.
Introduction
There is continuing interest in the development of improved methods for mapping brain function and organization. Functional MRI (fMRI) is one of the most important and useful of current techniques and is based on the sensitivity of MRI to changes in blood oxygenation, flow, and volume that accompany with changes in brain activation [Ogawa, 1990]. The spatial and temporal resolutions of this technique are limited by the nature of the coupling between neuronal electrical activity and the corresponding hemodynamic response. Spatial resolutions on the order of 1 mm and temporal resolutions on the order of 1 sec are readily achieved. There is considerable interest in developing methods to map neural events with greater temporal resolution.
Signal transfer along an axon is based on the ability of the membrane to alter its permeability to Na+ and K+ ions. These changes are caused by the opening of voltage-sensitive channels as a result of an approaching action potential. The action potential can be approximated by two oppositely oriented current dipoles whose separation depends on the conduction velocity. The magnitude of each dipole is about 100fAm. Although the precise natures of neural currents are complex, they generate weak magnetic fields within tissue that in principle may affect NMR signals [Cohen, 1984; Nunez, 2001; Bandettini, 2005]. One approach to detecting these fields is to try to measure the spatial displacement induced by neuronal electrical currents by the Lorentz effect [Song, 2001; Truong, 2006]. In addition, several groups have suggested that neural activity may be detected by MRI by measuring the signal losses and/or phase shifts produced by the local magnetic field perturbations associated with the local electric currents [Bodurka, 2002; Xiong, 2003]. This general approach has been termed magnetic source MRI (msMRI). Calculations predicting the magnitudes of these effects support the possibility that msMRI may be able to map neuronal activity in the human brain at high temporal and spatial resolution.
MR signals decrease when R2* (1/T2*) increases, which may arise whenever the nuclei within a voxel experience different magnetic fields for significant time intervals so that their NMR signals become dephased. In principle the local currents caused by neuronal activity could cause such effects. The msMRI protocol reported by Xiong et al 2003 measured the integrated activation within a short time frame (on the order of msec) [Xiong, 2003]. They showed that msMRI signals were measurable and equal to 1.12%±0.54% of the background MRI signal. They also tested the relationship between the msMRI signal strength and the echo time (TE). A nonlinear relationship between TE and msMRI magnitude was observed as predicted by a theoretical model. The data fit well with a quadratic current-dipole model of the magnetic fields induced by neuronal firing [Hobbie, 1997].
Unlike Bodurka’s methods [Bodurka, 1999; 2002], which were based on measuring net signal phase changes, the method of Xiong et al. was based on measuring changes in signal magnitude in standard fMRI techniques. Xiong et al. postulated that both positive and negative phases of MRI signals could destructively add, so that no net phase is detectable. On the other hand, the magnitude of the MRI signal may significantly change due to neuro-magnetic fields. Nuclear spins experiencing local field inhomogeneities produced by neural currents may lose phase coherence, resulting in a decrease of MRI signal magnitude. They proposed that magnitude measurements could be more sensitive for measuring in vivo neuronal activity than phase measurements. Experimentally, Chow et al. showed evidence of phase cancellation in the optic nerve and visual cortex, and demonstrated that only amplitude changes would be expected [Chow, 2004].
Neural activity involves the excitation of multiple neurons in complex patterns of firing and spiking. However, event-related electrophysiological studies have been explored for many years and several experimental paradigms in common use produce a relatively large amplitude, well defined surface potential at a specific time after a particular type of stimulus is presented. These so-called ERPs are often the results of a few discrete regions generating relatively large amounts of activity at specific times. We postulated that within a focal generator of an ERP, there should be significant neuronal currents that could induce MR signal dephasing at or around the time of the appearance of the surface ERP. We therefore looked for MR signal changes at the time of well known ERPs in those regions believed to be active in their production.
In the present study, we adapted the method of Xiong et al. [Xiong, 2003] to investigate whether we could detect an msMRI signal correlated in time and amplitude with two well defined event related potentials (ERPs), in locations previously identified as putative generators of the ERPs. The two ERPs investigated were the P300 produced by auditory oddballs, whose amplitude increases with decreasing frequency of the oddball event [Horovitz, 2002], and the face-sensitive N170, whose amplitude decreases with increasing noise level in the face presentation [Horovitz, 2004].
Method
The study was designed to identify regions of msMRI signal change associated with a well characterized brain activation paradigm, and to confirm that any signal changes observed are due to currents produced by neural activity. Our strategy was to use a paradigm in which the level of neural activity within a region could be modulated in a predictable manner, as measured by the electrical evoked response potential (ERP), and by subsequent event-related BOLD fMRI signal changes. We selected the auditory “oddball” paradigm and a face-sensitive paradigm, because it has been shown that the amplitude of the P300 and N170 responses can be modulated in a predictable manner.
Subjects
A total of 12 healthy volunteers (5 females, mean age 25.6, range 20–32 years) participated in the Auditory Oddball studies. Five of the subjects participated in the ERP experiments, five of the subjects in the BOLD-fMRI experiments, eight of the subjects in msMRI experiments and three of them participated in all experiments. Among these volunteers who participated in the Auditory Oddball studies, 4 healthy volunteers including 2 females with age range from 21 to 29 (mean age=25.5) also participated in the Face-Sensitive MRI studies.
All subjects had normal hearing and normal or corrected-to-normal vision. A written informed consent was obtained from all volunteers prior to the examinations. All subjects were instructed to maintain constant attention throughout all experiments.
Stimulus Presentation
Auditory oddball - Auditory stimuli generated by E-prime (Psychology Software Tools, Inc) were presented to healthy subjects every 1.2s. The frequent stimuli were 1kHz tones of 100ms duration, while rare stimuli -“oddballs”- were 1.5kHz tones of the same 100ms duration (Figure 1). The interval between oddballs was varied within runs to produce oddball presentation frequencies of 4%, 6% and 8% (Table 1). The different probabilities of oddballs were presented randomly. In total 50 oddballs for each presentation frequency were presented in 5 runs.
Figure 1.

Auditory oddball paradigm
Table 1.
Auditory oddball paradigm: the interval between oddballs varied to produce different oddball presentation frequencies.
| Tones between targets | TTI(seconds) | Probability(%) | #target/run |
|---|---|---|---|
| 12–13 | 15±0.6 | 8 | 10 |
| 16–17 | 19.8±0.6 | 6 | 10 |
| 24–25 | 29.4±0.6 | 4 | 10 |
Face processing - Visual stimuli generated by E-prime (Psychology Software Tools, Inc) were presented to healthy subjects for a period of 500 ms every 1.5s. In order to localize the area, before the event-related experiments, a block-design Face-Presentation experiment in which randomly intermixed block of 30s different faces presentation plus 20s fixations with block of 30s objects presentations plus 20s fixations were presented to obtain a sustained hemodynamic responses. The frequent stimuli were pictures of cars, while pictures of faces with different noise levels of 0%, 20% and 100% were presented every 19.5s (Figure 2) in event-related fashion. 24 face pictures were presented to the subject in one run.
Figure 2.
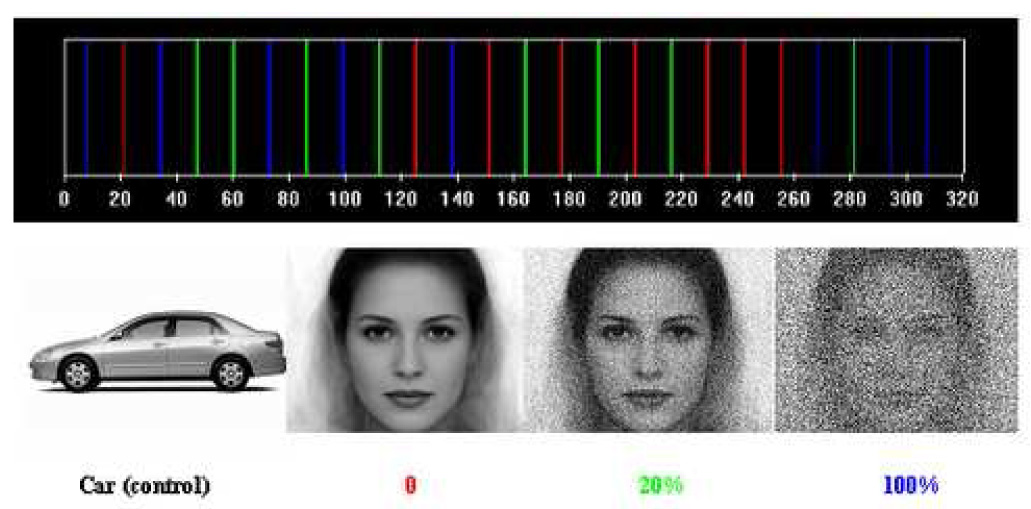
Face-presentation paradigm: black-car pictures were presented; red-0% noise added to the face picture; green-noisy image was created by adding gaussion noice with standard deviation of 20%; blue-noisy image was created by adding gaussion noise with standard deviation of 100%.
Data Acquisition
ERPs were collected from electrode Pz using an electrocap and recorded on a computer running Scan4.2 and controlling SynAmp amplifiers (Neuroscan, Inc). The temporal resolution was set to be 2msec, band pass filter 0.05–100 Hz and Gain is 500.
BOLD functional images were acquired on a 3T Philips Achieva (with a 6-channel head coil, SENSE factor = 2) using a gradient echo EPI sequence; slice thickness=5mm, gap=1mm, TE=35ms, flip angle=70°, FOV=22x22cm2 and acquisition matrix size=80×80 reconstructed to 128×128. 16 axial slices with TR=1.2s were acquired for the Auditory Oddball experiments, and 10 coronal slices with TR=1.5s for the block-design Face- Presentation experiments. A tool on the PHILIPS scanner console called iViewBold can describe simple block design paradigms and perform analysis while scanning and display correlation/activation maps in real-time. These real time activation maps are helpful for picking up the slice containing the region of interest.
For magnetic source MRI (msMRI) studies, the imaging parameters were the same except that only 1slice containing an ROI of interest was obtained. MR images of the slice including the supramarginal gyri were acquired at 325 or 425 or 525msec after presentation of the “oddball” in the P300 study. MR images of slices including the fusiform gyri were acquired with either 170msec or 180msec or 400msec delay relative to face stimulation onset in different runs. These delays were chosen in order to be able to study the time course of the T2*-weighted MR signal before and after the mean time of the maximum P300 or N170 ERP amplitudes (Figure 3).
Figure 3.

Magnetic Source MRT (msMRI) images were acquired with offsets relative to stimulation onsets.
Data Analysis
Scan4.2, SPM2, BrainVoyage QX and custom analysis package running under MATLAB were used for data analysis. A general linear transform model (GLM) [Friston 1995], which assumes that the fMRI signal possesses linear characteristics with respect to the stimulus and that the temporal noise is white, was used to estimate the response. We first used a correlation template generated by convolving the box-car function of the stimulation paradigm with a canonical fMRI impulse response function described by Friston et al. [Friston 1994] to do the cross-correlation analysis [Bandettini 1993]. Cross-correlation maps were thresholded (P < 0.001) to generate activation maps. Then a 1×3 voxel ROI was identified from the activated voxels. Within the ROI, a resting baseline was calculated for each “oddball” / face-presentation cycle by averaging 2 images obtained right before stimulus onset, then the percentage average intensity changes through time were calculated and showed as a time course.
Results
It was previously established that the N170 amplitude monotonically decreases as Gaussian noise is added to the picture of a face [Horovitz 2004]. We have also previously confirmed the inverse relationship between P300 amplitude and “oddball” frequency. The P300 amplitude decreases as “oddball” presentation frequency increases. Our studies confirmed the same behaviors (Figure 4).
Figure 4.
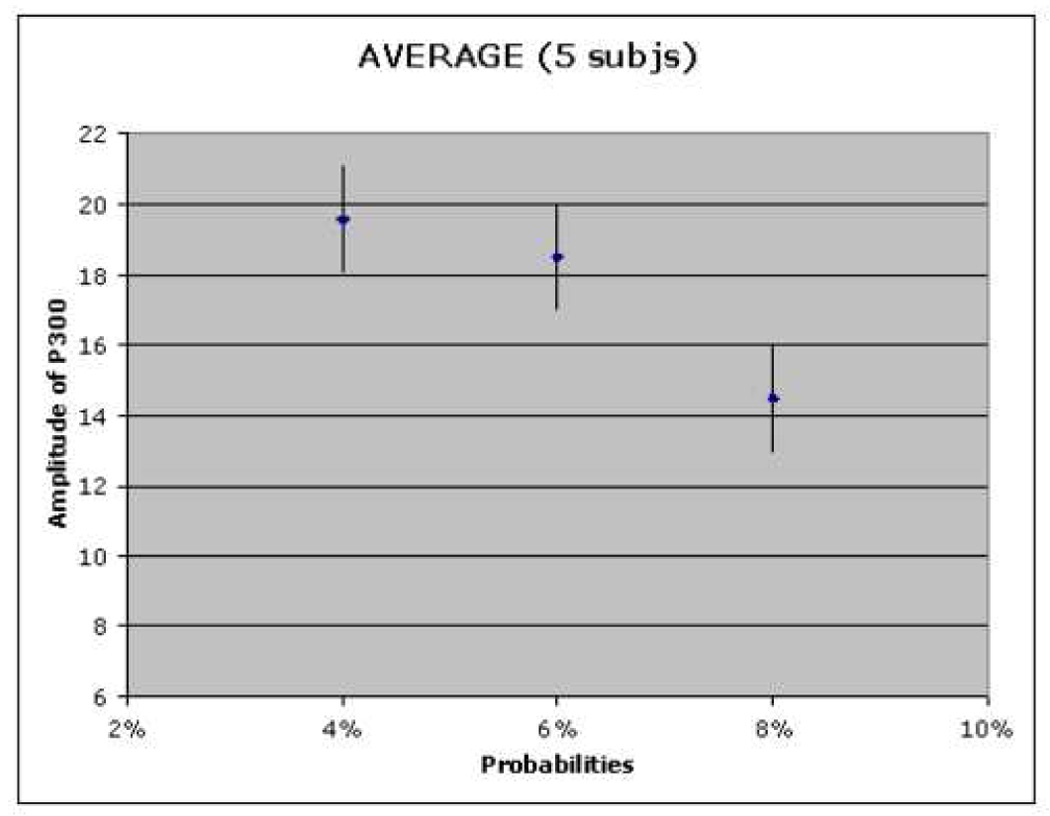
P300 amplitude decreases as “oddball” presented frequency increases.
Conventional event-related BOLD-fMRI analyses by cross-correlation analysis identified the same regions that showed covariations of BOLD signals and ERP amplitudes as previously described (Figure 5). For example the BOLD signals in the supramarginal gyrus (SMG) and anterior cingulate (ACG) [Tarkka, 1995 & 1998; Mulert 2004] and the P300 amplitude at Pz showed significant inverse correlations with the “oddball” frequency [Horovitz, 2003]. In addition, the BOLD signals in face fusiform gyrus (FFA) and the N170 amplitude showed inverse correlations with noise level [Horovitz, 2004].
Figure 5.
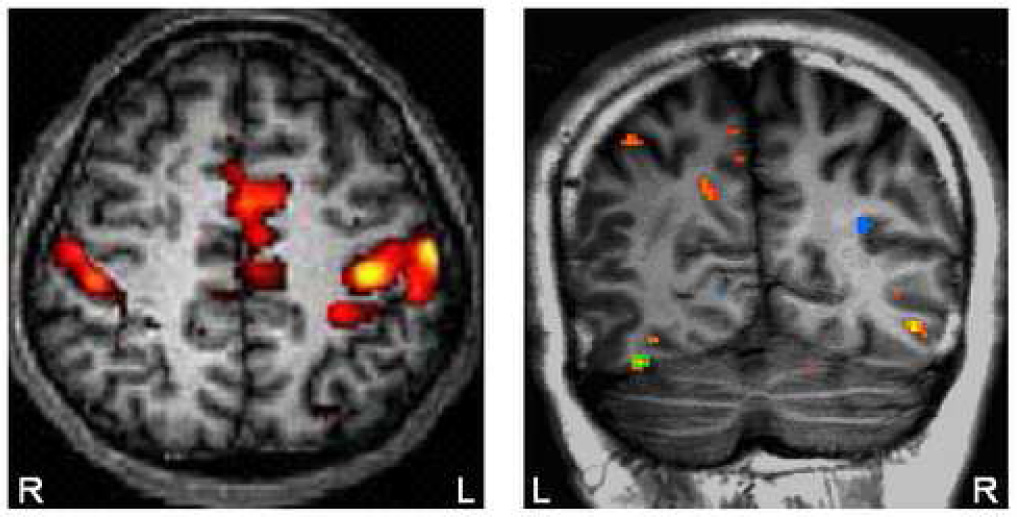
Left Panel: BOLD activation map for the auditory oddball experiment; right panel: Activation map for face-sensitive experiment P<0.001
Despite these robust BOLD activations which peaked several seconds after ERP, we have so far been unable to identify reliable msMRI signal amplitude changes at or near the timing of the ERPs that correlate with the corresponding ERP or BOLD data. Spatiotemporal activation maps (t-test) from a hybrid ms/BOLD fMRI study showed no significant signal loss (P<0.1) in SMG and ACG in auditory “oddball” studies for all 3 different offsets at 300~500ms after the oddballs. Early fMRI signal changes in FFA at different times after onset of face-presentation also showed no difference. Figure 6 shows the T2*-weighted signal time courses for N170 runs where images were synchronized to 170, 180 or 400 ms post stimulus, and shows no difference in the mean signal at any of those times, and no difference from the prestimulus signal. Similarly, no significant signal changes were seen at times corresponding to the P300 ERP in the auditory oddball condition.
Figure 6.
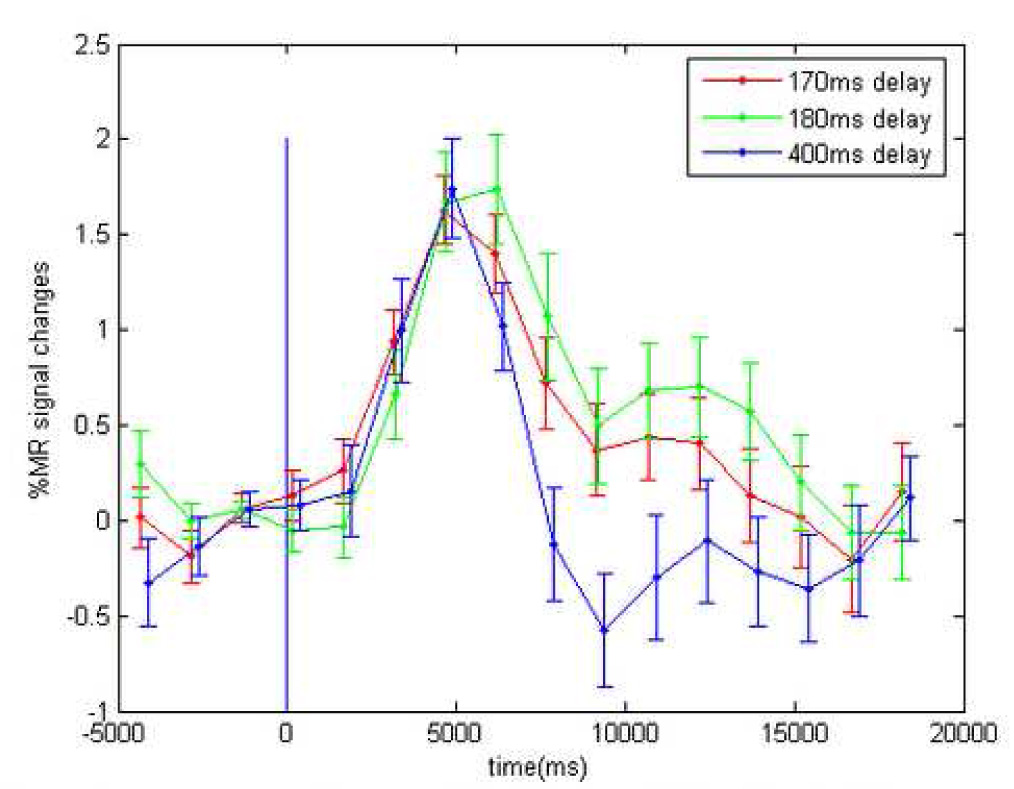
Time courses of functional MRI signals in ROI(FFC) with different offsets relative to the stimulus (face) onset. These are no significant difference in the mean signal at 170, 180 or 400 ms post stimulus, and no differences from the prestimulus signal.
Conclusion and Discussion
These experiments used an event-related design to probe the response of the T2*- weighted MRI signal at the time and in the locations of well-characterized ERP generators. We found no reliable evidence of changes in the MRI signal occurring at those times and locations when maximal neural activity (as judged by the surface ERP) is expected. These results suggest that the effects of neural dipole currents on the T2*- weighted signal which have been proposed as a direct measure of neural activity, lie below the level of detection at 3T using the paradigm investigated here, or that the maxima in these ERPs do not correspond to times of significant current-induced dephasing.
We aimed to establish whether msMRI is a reliable method that greatly increases the temporal resolution of MRI for detecting changes of neuronal activity with no compromise of spatial resolution. We were unable to identify reliable signal changes due to these stimuli at times other than when the BOLD hemodynamic effect has developed some seconds after the causal event.
While transient magnetic field effects have shown promise in studies performed in vitro [Bodurka, 2002; Konn, 2003; Park, 2004; Petridou, 2006], they have not been reliably validated in vivo. Various methods have been used to increase the detectability of magnetic fields. For example, others [Chu, 2004; Bianciardi, 2004; Parkes, 2007] introduced methods that can separate neuromagnetic field effects from BOLD effects, and they did not see significant effects related to magnetic field changes associated with human neuronal activity. Thus our study supports these other finds. The sensitivity of MRI for detecting amplitude changes produced by neuronal currents is too low to be practically useful.
Acknowledgments
We thank Silvina Horovitz for assistance in experimental design. Thanks also to Chris Cannistraci, Robin Avison and Donna Butler for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen D, Nemoto I. Ferromagnetic particles in the lung. Part I. The magnetizing process; IEEE Trans. Biomed. Eng. BME-31; 1984. pp. 261–273. [DOI] [PubMed] [Google Scholar]
- 3.Nunez PL, Wingeler BM, Silberstein RB. Spatial-temporal structures of human alpha rhythm: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Map. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Applied Magnetic Resonance. 2005;29(1):65–88. [Google Scholar]
- 5.Bodurka J, Jesmanowicz A, Hyde JS, Xu H, Estkowski L, Li SJ. Current-induced magnetic resonance phase imaging. J Magn Reson. 1999;137:265–271. doi: 10.1006/jmre.1998.1680. [DOI] [PubMed] [Google Scholar]
- 6.Bodurka J, Zhao X, Li SJ. Analysis of physical mechanisms of respiration-induced fMRI signal changes; Proc 8th Annual Meeting ISMRM; Denver. 2000. p. 1006. [Google Scholar]
- 7.Bodurka J, Bandettini PA. Toward Direct Mapping of neuronal Activity: MRI Detection of Ultraweak, Transient Magnetic Field Changes. Magnetic Resonance in Medicine. 2002;47:1052–1058. doi: 10.1002/mrm.10159. [DOI] [PubMed] [Google Scholar]
- 8.Song AW, Takahashi A. Lorentz effect imaging. Magnetic Resonance Imaging. 2001;19:763–767. doi: 10.1016/s0730-725x(01)00406-4. [DOI] [PubMed] [Google Scholar]
- 9.Truong TK, Song AW. Finding neuroelectric activity under magnetic-field oscillations (NAMO) with magnetic resonance imaging in vivo. Proc Natl Acad Sci U S A. 2006;103(33):12598–12601. doi: 10.1073/pnas.0605486103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong J, Fox PT, Gao JH. Directly Mapping Magnetic Field Effects of Neuronal Activity by Magnetic Resonance Imaging. Human Brain Mapping. 2003;20:41–49. doi: 10.1002/hbm.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow LS, Cook GG, Whitby E, Paley MN. Investigation of MR signal modulation due to magnetic fields from neuronal currents in the adult human optic nerve and visual cortex. Magn Reson Imaging. 2006 Jul;24(6):681–691. doi: 10.1016/j.mri.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Hobbie R. Intermediate physics for medicine and biology. New York: Springer-Verlag; 1997. pp. 136–227. [Google Scholar]
- 13.Horovitz SG, Skudlarski P, Gore JC. Correlations and dissociations between BOLD signal and P300 amplitude in an auditory oddball task: a parametric approach to combining fMRI and ERP. Magnetic Resonance Imaging. 2002;20(4):319–325. doi: 10.1016/s0730-725x(02)00496-4. [DOI] [PubMed] [Google Scholar]
- 14.Horovitz SG, Rossion B, Skudlarski P, Gore JC. Parametric design and correlational analyses help integrating fMRI and electrophysiological data during face processing. NeuroImage. 2004;22:1587–1595. doi: 10.1016/j.neuroimage.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Tarkka IM, Stokic DS, Basile LF, Papanicolaou AC. Electric source localization of the auditory P300 agrees with magnetic source localization. Electroencephalogr Clin Neurophysiol. 1995;96(6):538–545. doi: 10.1016/0013-4694(95)00087-f. [DOI] [PubMed] [Google Scholar]
- 16.Tarkka IM, Stokic DS. Source localization of P300 from oddball, single stimulus, and omittedstimulus paradigms. Brain Topogr. 1998;11(2):141–145. doi: 10.1023/a:1022258606418. [DOI] [PubMed] [Google Scholar]
- 17.Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, Juckel G, Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22(1):83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 18.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 19.Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Map. 1994;1:153–171. [Google Scholar]
- 20.Chu R, de Zwart JA, van Gelderen P, Fukunaga M, Kellman P, Holroyd T, Duyn JH. Hunting for neuronal currents: absence of rapid MRI signal changes during visual-evoked response. NeuroImage. 2004;23(3):1059–1067. doi: 10.1016/j.neuroimage.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Konn D, Gowland P, Bowtell R. MRI Detection of Weak Magnetic Fields Due to an Extended Current Dipole in a Conducting Sphere: A model for Direct Detection of Neuronal Currents in the Brain. Magnetic Resonance in Medicine. 2003;50:40–49. doi: 10.1002/mrm.10494. [DOI] [PubMed] [Google Scholar]
- 22.Bianciardi M, Di Russo F, Aprile T, Maraviglia B, Hagberg GE. Combination of BOLD-fMRI and VEP recordings for spin-echo MRI detection of primary magnetic effects caused by neuronal currents. Magn Reson Imaging. 2004;22(10):1429–1440. doi: 10.1016/j.mri.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Park TS, Lee SY, Park JH. Effect of nerve cell currents on MRI images in snall ganglia. Neuroreport. 2004;15:2783–2786. [PubMed] [Google Scholar]
- 24.Parkes LM, de Lange FP, Fries P, Toni I, Norris DG. Inability to directly detect magnetic field changes associated with neuronal activity. Magn Reson Med. 2007;57(2):411–416. doi: 10.1002/mrm.21129. [DOI] [PubMed] [Google Scholar]
- 25.Petridou N, Plenz D, Silva AC, Loew M, Bodurka J, Bandettini PA. Direct magnetic resonance detection of neuronal electrical activity. Proc Natl Acad Sci U S A. 2006;103(43):16015–16020. doi: 10.1073/pnas.0603219103. [DOI] [PMC free article] [PubMed] [Google Scholar]


