Abstract
In most clinical laboratories, low density lipoprotein (LDL) cholesterol is usually estimated indirectly with the Friedewald equation or directly with the N-geneous assay. We assessed LDL-cholesterol values obtained by both methods to find an appropriate fasting period and to assess the influence of the energy content of the last meal. Blood samples were taken from 28 healthy volunteers who had consumed a standard meal (107 g of carbohydrate, 658 kcal) followed by a fasting period of 12 and 18 h, or a high-energy meal (190 g of carbohydrate, 1011 kcal) with a fasting period of 12 h. Prolongation of the fasting period from 12 h to 18 h decreased glucose level, but did not decrease triacylglycerol, total cholesterol, or high density lipoprotein (HDL) cholesterol. LDL-cholesterol levels measured with the N-geneous assay did not change (94.0 ± 21.5 to 96.3 ± 19.1 mg/dl). LDL-cholesterol levels calculated with the Friedewald equation were also similar after fasting periods of 12 h (98.5 ± 21.4 mg/dl) and 18 h (99.7 ± 20.2 mg/dl). The high-energy meal did not change the level of LDL-cholesterol measured with the N-geneous assay (96.1 ± 21.2 mg/dl), or the glucose, triacylglycerol, total cholesterol, or HDL-cholesterol level, but LDL-cholesterol levels evaluated from the Friedewald equation (92.6 ± 20.3 mg/dl) became significantly lower. A fasting time longer than 12 h is not necessary to obtain reasonable blood lipid levels. The Friedewald equation gave higher LDL-cholesterol levels than N-geneous assay in young Japanese females who had eaten a low-energy meal, and lower values when they had eaten a high-energy meal. Thus, it may be necessary to pay attention to energy of nigh meal prior to blood withdrawal.
Keywords: diet, hyperlipidemia, human, lipid, nutrition
Introduction
A high level of low density lipoprotein cholesterol (LDL-cholesterol) is a major risk factor for the development of coronary heart disease [1–5]. LDL-cholesterol level has been estimated indirectly from measurements of total cholesterol, triacylglycerol, and high density lipoprotein cholesterol (HDL-cholesterol) by means of the Friedewald equation [6] in most clinical laboratories.
[LDL-cholesterol] = [total cholesterol] − [HDL-cholesterol] − [triacylglycerol]/5
The Friedewald equation was verified by comparing its results with those of a reference method known as beta-quantification, in which total LDL mass is directly measured by analytical ultracentrifugation [7, 8]. LDL-cholesterol concentration derived by use of the Friedewald equation is invalid when samples are collected in the non-fasting state or in the presence of increased triacylglycerol levels [8, 9]. The beta-quantification method requires complicated instrumentation including ultracentrifugation and polyanion-precipitation steps, and is not available for routine use in most laboratories. However, a new and relatively simple analytical method called N-geneous LDL-cholesterol assay was developed in 1998 by Rifai et al. [10–12]. The results obtained with this method correlated highly with those of beta-quantification assay, and met the established analytical performance goals recommended by the National Cholesterol Education Program (NCEP) [13]. Furthermore, LDL-cholesterol values obtained with the N-geneous assay are not affected by the presence of increased levels of triacylglycerol, unlike the Friedewald equation.
Since the Friedewald equation has still considerable advantages including simplicity and lack of cost, it seemed worthwhile to perform a detailed validation study of the Friedewald equation utilizing data obtained with the N-geneous assay as reference values. The influence of restrictions to the Friedewald equation, i.e., that blood should be sampled in the fasted state and that the triacylglycerol level should not be high, is also an important consideration. It still needs to be established clearly how long patients should fast before blood is drawn, and whether or not the energy content of the last meal influences the LDL-cholesterol value even though the blood is drawn in the fasted state.
Thus, we assessed values obtained with the Friedewald equation by comparing them with the levels obtained by use of the N-geneous assay, in order to determine an appropriate fasting period and to uncover the influence of the energy content of the last meal.
Methods
Subjects
Twenty-eight healthy Japanese female volunteers (20–23 years old) were recruited for this study. The body mass index (BMI) of the subjects was 20.4 ± 1.7 kg/m2. None was obese (BMI>kg/m2) but there were five lean subjects (BMI = 17.3 − 18.4 kg/m2) in this group. The body fat ratio was 25.4 ± 3.4%, as measured by using the bioimpedance analysis method (Tanita Body Fat Analyzer, model no. TBF-210, Tanita Co., Tokyo). This study was approved by The Human Research Ethics Committee at Tokyo University of Agriculture, and all participants gave written informed consent.
Blood sampling and clinical analysis
Blood samples were taken from the antecubital vein and analyzed by Mitsubishi Kagaku Bio-Clinical Laboratories, Inc., Tokyo. The following parameters were measured: glucose (Sica Liquid Glu, Kanto Kagaku, Tokyo), triacylglycerol (Determiner-c-Tg, Kyowa Medex, Tokyo), total cholesterol (Dia Auto T-Cho, Dia Reagents, Tokyo), HDL-cholesterol (Cholestest HDL, Daiichi Pure Chemicals Co. Ltd., Tokyo), and LDL-cholesterol (N-geneous assay, Daiichi Pure Chemicals Co. Ltd., Tokyo), LDL-cholesterol level was also estimated by using the Friedewald equation [6].
Meals
Two kinds of meals (standard meal and high-energy meal) were used. The standard meal has an energy content of 658 kcal and contains standard amounts of carbohydrate (107 g), protein (19 g), and fat (16 g), with 44 mg cholesterol, based on the 1998 National Nutrient State report, Japan [14]. The high-energy meal has an energy content of 1,011 kcal; its carbohydrate content was increased to 190 g, with unchanged levels of protein, fat and cholesterol. We increased only carbohydrate in the high-energy meal, because in preliminary trials, volunteers found difficulty to eat a high-energy meal with an increased fat content, complaining that it was too greasy. These meals corresponded to a common Japanese dinner menu, consisting of curry, rice, miso soup, and dessert.
Protocols
Two kinds of fasting period were examined. In the first trial (12 h fasting period), the subjects were asked to eat breakfast and lunch (around noon) as usual. Then all 28 volunteers were gathered in the dining room where meals were prepared by students majoring in nutritional science, started to eat together at seven o’clock in the afternoon, and were asked to fast for the following 12 h. At 8 am next morning, blood samples were withdrawn in the fasted state. After one week the second trial (18 h fasting period) was performed. The same subjects were asked to eat breakfast as usual around 8 am. Then they were given the standard meal at 1 pm and asked to fast for 18 h. At 8 am next morning, blood samples were withdrawn in the same way as in the first trial. In the third week, the third trial (high-energy meal) was performed. The same subjects were asked to eat breakfast and lunch (around noon) as usual. Then at 7 pm they ate the high-energy meal together in the dining room and were asked to fast for the following 12 h. At 8 am next morning, blood samples were withdrawn in the fasted state. Throughout all experimental periods, they were allowed to drink tap water or mineral water, even in the fasting periods.
Statistical analysis
All evaluated variables are presented as means ± SD. Statistical analysis was performed with the use of StatView512 (Ver1.2, BrainPower Inc., CA). Comparisons between 2 groups were done by paired t test Data were considered statistically significant at p<0.05.
Results
As shown in Table 1, first and second trials, prolongation of the fasting period from 12 h to 18 h decreased the glucose level from 83.6 ± 4.6 to 80.8 ± 6.4 mg/dl (p<0.05 paired t test). Triacylglycerol, total cholesterol, and HDL-cholesterol levels did not change. LDL-cholesterol levels measured with the N-geneous assay did not significantly change (from 94.0 ± 21.5 to 96.3 ± 19.1 mg/dl). LDL-cholesterol levels calculated by use of the Friedewald equation were also similar after fasting periods of 12 h (98.5 ± 21.4 mg/dl) and 18 h (99.7 ± 20.2 mg/dl). The LDL-cholesterol levels determined by the Friedewald equation were significantly higher than those by the N-geneous assay after both fasting periods. This finding is different from most previous results, which have indicated that the Friedewald equation underestimates LDL-cholesterol level [8, 9, 15].
Table 1.
Blood glucose and lipids levels in 28 subjects
| Trials | Meals | Fasting period (hours) | Glucose (mg/dL) | Triacylglycerol (mg/dL) | Total- cholesterol (mg/dL) | HDL- cholesterol (mg/dL) | LDL-cholesterol levels by N- geneous assay (mg/dL) | LDL-cholesterol levels from the Friedewald equation (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| 1st | Standard meal | 12 | 83.6 ± 4.60 | 63.6 ± 19.1 | 172.5 ± 23.5 | 61.3 ± 12.3 | 94.0 ± 21.5 | 98.5 ± 21.4* |
| 2nd | Standard meal | 18 | 80.8 ± 6.4# | 61.9 ± 23.4 | 173.8 ± 21.3 | 61.6 ± 11.7 | 96.3 ± 19.1 | 99.7 ± 20.2* |
| 3rd | high-energy meal | 12 | 83.7 ± 5.6 | 65.6 ± 21.9 | 168.31 ± 22.1 | 62.7 ± 11.7 | 96.1 ± 21.2 | 92.6 ± 20.3* |
#p<0.05 vs the 1st trial (paired t test)
*p<0.01 vs LDL-cholesterol levels by N-geneous assay (paired t test)
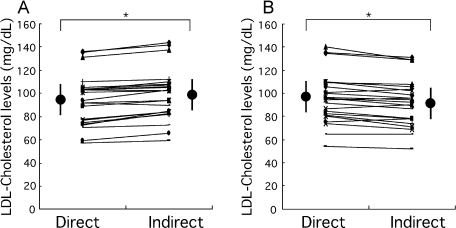
In the study to examine the influence of the energy content of the last meal on LDL-cholesterol levels, data obtained after a 12 h fasting period following the standard meal and the high-energy meal were compared (the first trial and the third trial of Table 1). Levels of glucose, triacylglycerol, total cholesterol, and HDL-cholesterol did not change. LDL-cholesterol level measured by the N-geneous assay did not change either (96.1 ± 21.2 vs 94.0 ± 21.5 mg/dl). However, that determined by the Friedewald equation became significantly lower in the high-energy meal trial (92.6 ± 20.3 vs 98.5 ± 21.4 mg/dl, p<0.05 paired t test). To examine these changes in detail, individual values of LDL-cholesterol obtained by the Friedewald equation and by the N-geneous assay were compared in the subjects given the standard meal (Fig. 1A) and the high-energy meal (Fig. 1B). In the case of the standard meal, LDL-cholesterol levels determined by the Friedewald equation were higher than the levels determined by the N-geneous assay in all but two subjects. In contrast, in the case of the high-energy meal, LDL-cholesterol levels determined by the Friedewald equation were lower than the levels determined by the N-geneous assay in all but four subjects. This indicates that the Friedewald equation underestimates LDL-cholesterol when the night meal prior to blood withdrawal is high in energy, but overestimates it when the night meal is low in energy.
Fig. 1.
Comparison between LDL-cholesterol levels determined by the N-geneous assay (Direct) and the Friedewald equation (Indirect) after a 12 h fasting period in 28 subjects. (A) After eating the standard diet (the first trial). (B) After eating the high-energy meal (the third trial). Values determined by the two methods in the same individuals are connected by lines. Large circles and bars indicate mean values and SD, respectively. *p<0.01 paired t test.
Discussion
High serum cholesterol is a major risk factor for coronary heart disease [1–5]. Serum cholesterol is distributed mainly among three major lipoprotein classes: very low density lipoproteins (VLDL), low density lipoproteins (LDL), and high density lipoproteins (HDL), with small amounts in intermediate density lipoproteins (IDL) and lipoproteins (a) [9, 15]. About 60–70% of cholesterol is contained in LDL. Thus, it is very important to evaluate LDL-cholesterol levels, as well as total cholesterol [9, 15–17]. To measure LDL-cholesterol directly, the LDL fraction in the serum has to be isolated by ultracentrifugation, followed by measurement of the cholesterol content. The process requires complicated technique and costly instrumentation [8]. In contrast, the Friedewald method does not require additional measurement other than total cholesterol, HDL-cholesterol, and triacylglycerol. In most epidemiological studies and in the general clinical setting [18, 19], LDL-cholesterol value is obtained by use of the Friedewald equation. This equation was validated by Warnick et al. [8], who confirmed that the equation is valid when the triacylglycerol level is less than 400 mg/dl. More detailed validation studies have not been performed because the beta-quantification method is not available in routine clinical settings. However, even in countries where people used not to favor lipid-rich foods, dietary habits are rapidly becoming Westernized, and the morbidity rate of coronary heart disease is increasing [20]. Consequently, the use of the Friedewald equation is becoming even more widespread, because of its simplicity and lack of cost. Since the N-geneous assay is more convenient than the beta-quantification method, we employed it to investigate two undefined issues regarding the Friedewald equation. The first of these was the appropriate fasting period. We found that the values after 18 h of fasting were not significantly different from those after 12 h (Table 1). Therefore, 12 h is a sufficient fasting period. Consumption of a high energy meal prior to fasting did not change the mean value of LDL-cholesterol determined by N-geneous assay compared with the control diet, but it significantly reduced the LDL-cholesterol level obtained by use of the Friedewald equation (Table 1). This is consistent with reported findings that the Friedewald method underestimates LDL-cholesterol [13, 15]. Even at low cholesterol levels, underestimation of LDL-cholesterol occurs [21]. Thus, the overestimation of LDL-cholesterol level by the Friedewald equation in the standard meal trial was puzzling. Since triacylglycerol was not significantly affected by the energy content of the previous meal in this study, the reason for the increase or decrease in LDL-cholesterol obtained with the Friedewald equation is obscure. One possible reason might be the low triacylglycerol levels and young age of the subjects in this study. Another reason may be that the Japanese standard energy content meal might have had a lower energy content than meals given in other countries in which underestimation of the Friedewald equation was reported [9, 15, 21]. Further study is needed to see whether this result is peculiar to Japanese young females or can be generalized to other groups.
Conclusion
Prolongation of fasting time beyond 12 h is not necessary for determination of blood lipid levels. The Friedewald equation gave higher LDL-cholesterol levels than N-geneous assay in young Japanese females who had eaten a low-energy meal before fasting, and lower values when they had eaten a high-energy meal. Thus, it may be necessary to pay attention to energy of night meal prior to blood withdrawal.
References
- 1.Gordon T., Kannel W.B., Castelli W.P., Dawber TR. Lipoproteins, cardiovascular disease, and death. The Framingham Study. Arch. Intern. Med. 1981;141:1128–1131. [PubMed] [Google Scholar]
- 2.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 3.The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–374. [PubMed] [Google Scholar]
- 4.Rifkind B.M., Lenfant C. Cholesterol lowering and the reduction of coronary heart disease risk. JAMA. 1986;256:2872–2873. [PubMed] [Google Scholar]
- 5.Grundy S. Role of low-density lipoproteins in atherogenesis and development of coronary heart disease. Clin. Chem. 1995;41:139–146. [PubMed] [Google Scholar]
- 6.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 7.Havel R., Eder H., Bragdon J. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Inves. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warnick G.R., Knopp R.H., Fitzpatrick V., Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990;36:15–19. [PubMed] [Google Scholar]
- 9.Hata Y., Mabuchi H., Saito Y., Itakura H., Egusa G., Ito H., Teramoto T., Tsushima M., Tada N., Oikawa S., Yamada N., Yamashita S., Sakuma N., Sasaki J. Report of the Japan Atherosclerosis Society (JAS) Guideline for Diagnosis and Treatment of Hyperlipidemia in Japanese adults. J. Atheroscler. Thromb. 2002;9:1–27. doi: 10.5551/jat.9.1. [DOI] [PubMed] [Google Scholar]
- 10.Rifai N., Iannotti E., DeAngelis K., Law T. Analytical and clinical performance of a homogeneous enzymatic LDL-cholesterol assay compared with the ultracentrifugation-dextran sulfate-Mg2+ method. Clin. Chem. 1998;44:1242–1250. [PubMed] [Google Scholar]
- 11.Nauck M., Warnick G.R., Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin. Chem. 2002;48:236–254. [PubMed] [Google Scholar]
- 12.Miller W.G., Waymack P.P., Anderson F.P., Ethridge S.F., Jayne E.C. Performance of four homogeneous direct methods for LDL-cholesterol. Clin. Chem. 2002;48:489–498. [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics, author. National Cholesterol Education Program: Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:525–584. [PubMed] [Google Scholar]
- 14.The Study Circle for Health and Nutrition Information. Recommended dietary allowance for Japanese, 6th ed. Dai-ichi Shuppan Publishing Co. Ltd.; Tokyo: 1999. pp. 1–273. (in Japanese) [Google Scholar]
- 15.Warnick G.R., Wood P.D. National Cholesterol Education Program recommendations for measurement of high-density lipoprotein cholesterol: executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin. Chem. 1995;41:1427–1433. [PubMed] [Google Scholar]
- 16.Levy R.I. Changing perspectives in the prevention of coronary artery disease. Am. J. Cardiol. 1986;57:17G–26G. doi: 10.1016/0002-9149(86)90661-2. [DOI] [PubMed] [Google Scholar]
- 17.Bush T.L., Fried L.P., Barrett-Connor E. Cholesterol, lipoproteins, and coronary heart disease in women. Clin. Chem. 1988;34:B60–70. [PubMed] [Google Scholar]
- 18.Özsoy M.B., Pabuçcuoglu A. The effect of acetaminophen on oxidative modification of low-density lipoproteins in hypercholesterolemic rabbits. J. Clin. Biochem. Nutr. 2007;41:27–31. doi: 10.3164/jcbn.2007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed K.A., Muniandy S., Ismail I.S. Role of Nε-(carboxymethyl)lysine in the development of ischemic heart disease in type 2 diabetes mellitus. J. Clin. Biochem. Nutr. 2007;41:97–105. doi: 10.3164/jcbn.2007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egusa G., Yamane K. Lifestyle, serum lipids and coronary artery disease: comparison of Japan with the United States. J. Atheroscler. Thromb. 2004;11:304–312. doi: 10.5551/jat.11.304. [DOI] [PubMed] [Google Scholar]
- 21.Scharnagl H., Nauck M., Wieland H., Marz W. The Friedewald formula underestimates LDL cholesterol at low concentrations. Clin. Chem. Lab. Med. 2001;39:426–431. doi: 10.1515/CCLM.2001.068. [DOI] [PubMed] [Google Scholar]