Abstract
Ischemia-reperfusion injury associated with liver transplantation remains a serious complication in clinical practice. In the present study the effect of intake of α-tocopherol or β-carotene to limit liver injury by oxidative stress in ischemia and reperfusion was explored. Wistar rats were fed with diets enriched with α-tocopherol (20 mg/day) or β-carotene (3 mg/day) for 21 days. After 21 days, their livers were subjected to 15 and 30 min of ischemia and afterwards were reperfused for 60 min. The recovery of levels of ATP during reperfusion was better in the group of rats whose diets were supplemented with α-tocopherol or β-carotene than in the group control. The supplementation of the diet induced changes in the profile of enzymatic antioxidants. The supplementation with α-tocopherol and β-carotene resulted in a decreased of superoxide dismutase during the ischemia and a recovery was observed after reperfusion. Not changes were observed for the enzymes catalase and glutathione peroxidase and glutathione but their values were higher to those of the group control. In conclusion, the supplementation with α-tocopherol and β-carotene improve the antioxidant and energetic state of liver after ischemia and reperfusion injury.
Keywords: oxidative stress, GSH, α-tocopherol, β-carotene, ischemia/reperfusion
Introduction
Epidemiological studies have shown that nutritional antioxidants slow down the progression and consequences of the diseases associated to oxidative stress. Diets rich in vitamins with antioxidant capacity have been of interest because of their potential health benefit against cardiovascular diseases, cancer, transplantation tissues, etc [1, 2].
Total interruption of hepatic blood flow is sometimes necessary during surgery, and it is indispensable in hepatic transplantation. Liver transplantation provides effective therapy for most forms of acute and chronic liver failure, ischemia-reperfusion (I/R) injury, inherent in every liver transplantion, is the main cause of both initial poor function and primary non-function of liver allograft [3]. This involves a period of ischemia followed by reperfusion. It is well established that the liver is quite sensitive to ischemia [3, 4], but an additional component of injury could result from events occurring during reperfusion [4]. A large number of factors and mediators play a part in liver I/R injury [5–7]. Early activation of Kupffer cells enhances the formation of reactive oxygen species (ROS) and proinflammatory mediators such as tumor necrosis factor-α, interkeukin-1 and proteases [8]. The activation and migration of the polymorphonuclear leukocytes (PMN) at the target site prolong the deleterious process by secreting inflammatory cytokines, adhesion molecules, and reactive oxygen species [3, 9, 10]. The enhancer formation of reactive oxygen is considered to be responsible for triggering a severe oxidative stress. Toxicity of ROS arises from the peroxidation of membrane phospholipids, which may cause membrane disintegration resulting in mitochondrial dysfunction and loss of the energetic homeostasis [11].
Antioxidants are substances that react with oxygen free radicals and stop tissue oxidation [12, 13]. The endogenous antioxidant system consists mainly of enzymes such as catalase, superoxide dismutase, and glutathione peroxidase. Sometimes is necessary the action of exogenous antioxidants such as vitamins α-tocopherol, ascorbic acids and the carotenoids [12] to reinforce the endogenous antioxidant capacity.
α-Tocopherol and β-carotene have been widely studied because of their special chemical and nutritional properties. Both antioxidants are essential components found in the normal mammalian diet, and oral supplementation with these compounds is well tolerated. Therefore, α-tocopherol and β-carotene provide a first line of defence against DNA oxidative damage and act mainly as a chain-breaking antioxidant lipid peroxidation of unsaturated fatty acids in the cell membrane [14–17].
In the present study, we determine the cellular status in relation to antioxidative defences in an experimental model of ischemia/reperfusion in rat liver. The preventive effect on the oxidative stress by the administration of antioxidants (i.e. α-tocopherol or β-carotene) with the diet was studied measuring the ATP levels, antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX)) and glutathione (GSH) levels.
Materials and Methods
Chemicals and reagents
All reagents were of analytical grade and obtained from Merck (Darmstadt, Germany) and Scharlau (Barcelona, Spain). Enzymes and coenzymes together with other substrates used in this study were of the highest grade and available from comercial Boehringer (Mannhein, Germany) and Sigma Chemical Co. (St. Louis, MO). Standard diets were prepared by Letica (Barcelona, Spain)
Animals
Adult male Wistar rats, weighing from 170–250 g, were used in the present study. They were maintained on a 12 h day-night rhythm with free access to water and food. The animals were fasted for 24 h before surgery, but allowed water ad libitum. The experiment was performed in accordance with the Recommendations of the Helsinki Declaration.
Experimental design and protocol
The animals were randomly divided into three main experimental groups: control, α-tocopherol and β-carotene treated comprising of ten animals each group. Animals of control group were fed with a basal diet (Letica, Barcelona); α-tocopherol group were supplemented with α-tocopherol (20 mg/day) once orally for 21 days; β-carotene groups were supplemented with β-carotene (3 mg/day) once orally also during 21 days.
Induction of ischemia-reperfusion injury
Anaesthesia was induced intraperitoneally with pentobarbitone sodium at doses 60 mg/Kg. After laparotomy, liver was perfused in situ in a non-recirculating system in the anterograde direction (via portal vein), with a Krebs-Henseleit bicarbonate buffer supplemented with EGTA (0.5 mM) and glucose (10 mM) saturated with O2/CO2 95/5 (v/v), pH 7.4 at 4°C. After cannulating the portal vein, livers were perfused for 5 min and tissue samples were taken, freeze-clamped and immediately frozen in N2 liquid (initial time). Liver ischemia was induced by interruption of the flow while the system was kept at 4°C. At time 15 and 30 min of liver ischemia samples were cut, freeze clamped and frozen in N2. The reperfusion was allowed for 60 min with the Krebs-Henseleit oxygenated buffer supplemented with CaCl2 (1.25 mM) at 37°C. Initial flow rate of reperfusion was kept moderately low (2 ml/min/g of liver) and increased gradually to 4 ml/min/g of liver over 4 to 5 min and tissue sample was also obtained (reperfusion 60 min). At the end 4 samples into each group was obtained: initial, ischemia 15 min (Is 15), ischemia 30 min (Is 30) and reperfusion 60 min (rep 60).
ATP determination
At the end of the incubation period metabolic reactions were stopped by the addition of 0.4 mL of 20% (v/v) perchloric acid to the incubation mixture and placed on ice-containing tray. Acidified incubated samples were centrifuged to precipitate proteins and the supernatants were neutralized with potassium hydroxide for the enzymatic determination of ATP. The ATP levels were determined measuring spectrophotometrically at 340 nm the NADPH oxidation in the presence of glucose [18].
Measurement of antioxidant enzymes activities
The activity of the total superoxide dismutase (SOD) was according to the method of McCord and Fridovich [19] based on the production of superoxide radicals during the conversion of xanthine to uric acid by xanthine oxidase and the inhibition of cytochrome c reduction. One unit of SOD activity was defined as the amount of SOD that produces 50% inhibition of cytochrome c reduction.
Catalase activity was assayed using the method described by Clairbone [20]. The reaction mixtures (1 mL) consisted of 50 mM potassium phosphate (pH: 7.0) 19 mM H2O2 and a sample. The reaction was initiated by the addition of H2O2 and absorbance changes were measured at 240 nm.
Glutathione peroxidase activity (GPx) was determined measuring the conversion of NADPH to NADP in the presence of reduced glutathione (GSH) and H2O2 spectrophotometrically at 340 nm according to the method described by Flohe and Gunzler [21].
Measurement of GSH levels
The levels of total GSH have been determined by the assay with glutathione-s-transferase according to Brigelius et al. [22].
Statistics
Results are expressed as mean values ± SD. Comparison of the means of four measurements using a significance level of p<0.05 was performed by one-way analysis of variance (ANOVA) using the Statgraphics Computer System.
Results
Table 1 shows the ATP levels at initial time, after 15 and 30 min of ischemia (Is) and after 60 min of reperfusion (Rep) of hepatocytes isolated from control group rats and of the rats whose diet were supplemented with α-tocopherol or β-carotene. In control rats ATP levels were of 1.05 ± 0.15 µmol/g tissue for initial time and the levels decreased significantly to values of 0.29 ± 0.07 µmol/g tissue and 0.14 ± 0.04 µmol/g tissue after 15 and 30 min of ischemia. A little but not significant recovery of the ATP levels occurred after reperfusion at 60 min. In the groups of the rats that took α-tocopherol and β-carotene the ATP levels decrease significantly during the ischemia but after 60 min of reperfusion the levels increased significantly to recovery initial values. When compared ATP levels of hepatocytes of rats that took a diet supplemented with α-tocopherol o β-carotene ATP with control group, not differences were observed at initial time and after 15 of ischemia. However, levels significantly higher were observed after 30 min of ischemia and of 60 of reperfusion in the group of rats that took α-tocopherol or β-carotene.
Table 1.
Concentrations of adenosin triphosphate levels (ATP) in the liver of the different groups: control, α-tocopherol group and β-carotene group.
| ATP (µmol/g tissue) | Control | α-tocopherol | β-carotene |
|---|---|---|---|
| Initial | 1.052 ± 0.15b | 0.975 ± 0.27b | 1.00 ± 0.3b |
| Is 15 min | 0.291 ± 0.068a | 0.238 ± 0.117a | 0.358 ± 0.17a |
| Is 30 min | 0.142 ± 0.039a | 0.210 ± 0.097a* | 0.297 ± 0.06a* |
| Rep 60 min | 0.362 ± 0.025a | 0.880 ± 0.188b* | 0.852 ± 0.17b* |
ATP levels were measured at initial time (control), after 15 (Is 15 min) and 30 (Is 30 min) minutes of ischemia and after 60 min of reperfusion (Rep 60 min). Results are expressed as mean ± SD of 10 separated experiments. For the same diet values with different alphabetical letters are significantly different (p<0.05). * Indicates that values are different (p<0.05) vs to control group.
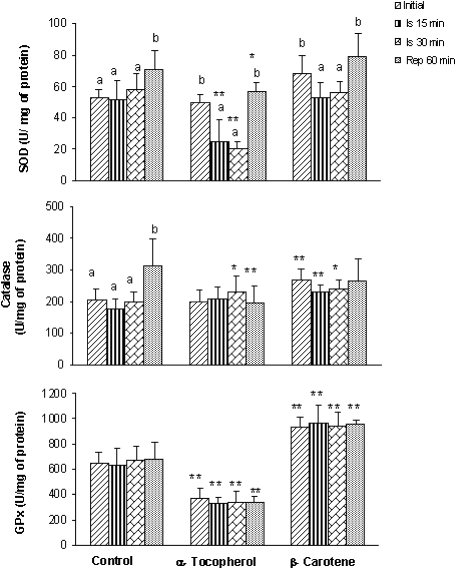
The effect of the supplemented diet on endogenous enzymatic activities (SOD, catalase and GPx) was studied (Fig. 1). In the control group the activity of the three enzymes not change significantly during the ischemia but an increase after 60 min of reperfusion for SOD and catalase activity was observed. The group of the rats fed with an enriched diet in α-tocopherol or β-carotene showed a significant decrease in SOD activity during ischemia periods (15 and 30 minutes, p<0.05), with a recovery in the reperfusion period that reached the initial values. In relation to catalase and GPX activities no significant differences were observed among initial, ischemia and reperfusion time.
Fig. 1.
Superoxide dismutase, catalase and glutathione peroxidase levels in homogenate rat liver, fed with different diets: control diet, diet supplemented with α-tocopherol and diet supplemented with β-carotene. Enzyme activities were measured at initial time, after 15 (Is 15 min) and 30 (Is 30 min) minutes of ischemia and after 60 min of reperfusion (Rep 60 min). Results are expressed as mean ± SD of 10 separated experiments. For the same diet values with different alphabetical letters are significantly different (p<0.05). * Indicates that values are different (p<0.05) vs to control group.
When compared the groups of rats whose diet were supplemented with α-tocopherol and β-carotene with the control group we observed that to initial time the group of α-tocopherol showed lower values of GPx activity, but the β-carotene group showed for all enzymes higher activities. For ischemia and reperfusion period, the enzymatic activities in the group of α-tocopherol are lower than those of the control group. Nevertheless, the catalase and GPx activities of β-carotene group were higher than those of the control group.
Pattern of changes in GSH levels during I/R can be seen in Table 2, not changes were observed in control and β-carotene group. However, the group of the rats that took α-tocopherol, a significant increase (p<0.005) in GSH values was observed at 30 min of ischemia time. When compared the GSH levels among groups, we observed that the groups that took α-tocopherol and β-carotene showed values higher that those of the control group.
Table 2.
Glutathione (GSH) levels in homogenate rat liver, fed with different diets: control diet, diet supplemented with α-tocopherol and diet supplemented with β-carotene.
| GSH (µmol/g tissue) | Control | α-tocopherol | β-carotene |
|---|---|---|---|
| Initial | 2.28 ± 0.237 | 4.11 ± 0.448ab* | 3.55 ± 0.738* |
| Is 15 min | 2.26 ± 0.260 | 4.05 ± 0.609ab* | 3.21 ± 0.433* |
| Is 30 min | 2.19 ± 0.392 | 4.25 ± 0.855b* | 3.67 ± 0.649* |
| Rep 60 min | 2.29 ± 0.195 | 3.64 ± 0.269a* | 3.12 ± .711* |
GSH levels were measured at initial time (control), after 15 (Is 15 min) and 30 (Is 30 min) min of ischemia and after 60 min of reperfusion (Rep 60 min). Results are expressed as mean ± SD of 10 separated experiments. For the same diet values with different alphabetical letters are significantly different (p<0.05). *Indicates that values are different (p<0.05) to control group. **Indicates that values are different (p<0.005) to control group.
Discussion
Oxidative stress is an imbalance between oxidants such as ROS and antioxidants, and probably contributes to the development, progression and complications of ischemia-reperfusion, which is characterized by the increased production or decreased elimination of antioxidants [3]. Epidemiological and clinical studies suggest that dietary antioxidants may reduce the risk of different diseases and is often associated with several health benefits correlated with their antioxidant properties [2]. α-tocopherol and β-carotene reduces the level or reactive oxidant species intracellular and reacts rapidly with a variety of oxidants [15].
Preservation of the liver in transplantation procedures involves a period of ischemia. Mechanisms for induced ischemia injury are not completely clarified, but they could be related to depressed mitochondrial function and a reduced capacity to regenerate high-energy phosphate compounds (ATP) by the liver [3, 23]. During the ischemia period of liver transportation and preservation, maintenance of energy charge and adequate nucleotide levels are critical for tissue viability because the high metabolic rate of hepatic tissue makes it vulnerable to the injurious effects of ischemia [24]. In our study it was observed that the recovery of tissue ATP levels after reperfusion in the rats fed with a diet enriched with α-tocopherol or β-carotene. The restorative effect of α-tocopherol and β-carotene on ATP and energy metabolism is explained by the antioxidant and membrane protective action of these compounds on mitochondrial functions [25, 26]. The antioxidant capacity of vitamin E resides in transferring a phenolic H+ to oxidant radicals derived from oxidized polyunsaturated fatty acids [13, 14, 17]. β-carotene may interact with free radicals by electron transfer, hydrogen abstraction and addition of a radical species and it is very reactive to peroxyl radicals [27].
Some intracellular enzymes, e.g. superoxide dismutase, catalase and glutathione peroxidase can effectively block the activity of the free oxygen radicals and thus keep the normal biomolecules peroxidation rate [2]. In our study not changes were observed during the ischemia but in the reperfusion the influx of oxygenated blood was accompanied by a stimulation of superoxide dismutase and catalase activities. Some naturally antioxidants, such as α-tocopherol and β-carotene can play a role as active scavengers of free radicals and modified the levels of enzymes antioxidants. At the present time, the effect of α-tocopherol or β-carotene on the activity of antioxidants enzymes in I/R has not been clarified.
In the rats that took α-tocopherol or β-carotene hepatic superoxide dismutase activity decreased significantly at 15 and 30 min of ischemia compared to initial time and recovery initial values after 60 min of reperfusion, but not changes were observed for catalase and GPx activities. This decreased could be due to their role as scavengers of superoxide radical or as inhibitors of their production. Previous studies have demonstrated an increase in superoxide production in Kupffer cells isolated from ischemia and reperfused livers [3].
When the α-tocopherol group was compared with control group the superoxide dismutase after ischemia and reperfusion period, catalase after reperfusion and glutathione peroxidase in all periods showed lower activities than those of control group. In contrast, the β-carotene group showed higher activities for all enzymes than those of control group.
The reason of the changes observed in enzymes activities result of the supplementation is not clear. α-tocopherol may act as direct scavenger of reactive oxygen species mainly of superoxide radical and decrease the hepatic need for certain antioxidant enzymes. In contrast, β-carotene could increase the antioxidant enzyme activities in response to an increase in oxidative stress. It is known that the expression of antioxidant enzymes are induced and regulated by ROS and apparent prooxidant effects of carotenoids have been reported in vitro by different causes [27–29]. One of the enzymes that increase significantly in the rats fed with β-carotene is the GPx. These results suggest that these rats to have a stronger capacity to protect the peroxidation of the membrane and that would explain the protective effect observed on ATP levels during the reperfusion.
GSH participates in the antioxidative defence in different ways [30–33]. Although no significant changes were observed between the ischemia and reperfusion period in either the standard or supplemented diet, our results show higher values of GSH in the latter one. GSH depletion of about 20 to 30% of total glutathione levels can impair the cell’s defence against the toxic actions of such compounds and may lead to cell injury and death [34, 35]. In this sense, the maintenance of high levels of GSH is crucial for the surviving of the cells [35].
Conclusions
In summary, our results indicate that the supplementation with α-tocopherol and β-carotene increase the levels of ATP during the reperfusion possibly as a result of the changes observed in the enzymatic (SOD, catalase and GPx) and no-enzymatic (GSH) antioxidant state of the liver.
Acknowledgment
We are grateful to Yolanda Fernandez for revising the English text.
References
- 1.Halliwell B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 2.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell, B. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Casillas-Ramirez A., Ben M.I., Ramalho F., Rosello-Catafau J., Peralta C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006;79:1881–1894. doi: 10.1016/j.lfs.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Peralta C., Bulbena O., Xaus C., Prats N., Cutrin J.C., Poli G., Gelpi E., Rosello-Catafau J. Ischemic preconditioning: A defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002;73:1203–1211. doi: 10.1097/00007890-200204270-00004. [DOI] [PubMed] [Google Scholar]
- 5.Banga N.R., Homer-Vanniasinkam S., Graham A., Al-Mukhtar A., White S.A., Prasad K.R. Ischaemic preconditioning in transplantation and major resection of the liver. British J. Surgery. 2005;92:528–538. doi: 10.1002/bjs.5004. [DOI] [PubMed] [Google Scholar]
- 6.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. 2003; 284:15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 7.Serracino-Inglott F., Habib N.A., Mathie R.T. Hepatic ischemiareperfusion injury. Am. J. Surgery. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 8.Nakamitsu A., Hiyama E., Imamura Y., Matsuura Y., Yokoyama T. Kupffer cell function in ischemic and nonischemic livers after hepatic partial ischemia/reperfusion. Surg. Today. 2001;31:140–148. doi: 10.1007/s005950170198. [DOI] [PubMed] [Google Scholar]
- 9.Decker K. Biologically-Active Products of Stimulated Liver Macrophages [Kupffer Cells] Eur. J. Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H. Mechanisms of Oxidant Stress-Induced Acute Tissue-Injury. Proc. Soc. Exp. Biol. Med. 1995;209:104–111. doi: 10.3181/00379727-209-43885b. [DOI] [PubMed] [Google Scholar]
- 11.Rosser B.G., Gores G.J. Liver-Cell Necrosis—Cellular Mechanisms and Clinical Implications. Gastroenterology. 1995;108:252–275. doi: 10.1016/0016-5085(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 12.Lachance P.A., Nakat Z., Jeong W.S. Antioxidants: An integrative approach. Nutrition. 2001;17:835–838. doi: 10.1016/s0899-9007(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 13.Thomas M.J. The role of free radicals and antioxidants. Nutrition. 2000;16:716–718. doi: 10.1016/s0899-9007(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 14.Blatt D.H., Leonard S.W., Traber M.G. Vitamin E kinetics and the function of tocopherol regulatory proteins. Nutrition. 2001;17:799–805. doi: 10.1016/s0899-9007(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 15.McCall M.R., Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic. Biol. Med. 1999;26:1034–1053. doi: 10.1016/s0891-5849(98)00302-5. [DOI] [PubMed] [Google Scholar]
- 16.Chow B., Jacobson J.M. Biologically inspired molecular assembly lines. Bt Technol. J. 2004;22:270–277. [Google Scholar]
- 17.Martin A., Janigian D., Shukitt-Hale B., Prior R.L., Joseph J.A. Effect of vitamin E intake on levels of vitamins E and C in the central nervous system and peripheral tissues: implications for health recommendations. Brain Res. 1999;845:50–59. doi: 10.1016/s0006-8993(99)01923-x. [DOI] [PubMed] [Google Scholar]
- 18.Lamprecht W. In: Adenosin triphosphate [ATP], determination with hexokinase and glucose-6-phodphate dehydrogenase, in Methods of Enzymatic Analysis. Bergmeyer H.V., editor. Academic Press; New York: 1976. pp. 570–577. [Google Scholar]
- 19.McCord M., Fridovich. I. In: Cytocrome C, in CRC Handbook of methods for oxygen radicals research. Greenwald R.A., editor. Boca Ratón; Florida: 1986. pp. 213–214. [Google Scholar]
- 20.Clairbone A. In: Catalase activity, in CRC Handbook of methods for oxygen radicals research. R.A. Greenwald., editor. Boca Ratón; Florida: 1986. pp. 283–284. [Google Scholar]
- 21.Flohe L., Gunzler W.A. In: Glutathione peroxidase, in CRC Handbook of methods for oxygen radicals research. Greenwald R.A., editor. Boca Ratón; Florida: 1986. pp. 285–290. [Google Scholar]
- 22.Brigelius R., Muckel C., Akerboom T.P.M., Sies H. Identification and Quantitation of Glutathione in Hepatic Protein Mixed Disulfides and Its Relationship to Glutathione Disulfide. Biochem. Pharmacol. 1983;32:2529–2534. doi: 10.1016/0006-2952(83)90014-x. [DOI] [PubMed] [Google Scholar]
- 23.Ilhan N., Halifeoglu I., Ozercan H.I., Ilhan N. Tissue malondialdehyde and adenosine triphosphatase level after experimental liver ischaemia-reperfusion damage. Cell Biochem. Funct. 2001;19:207–212. doi: 10.1002/cbf.912. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H., Jin M.B., Ogata T., Taniguchi M., Suzuki T., Shimamura T., Magata S., Horiuchi H., Ogata K., Masuko H., Fujita M., Furukawa H., Todo S. Role of cyclic nucleotides in ischemia and reperfusion injury of canine livers. Transplantation. 2002;73:1041–1048. doi: 10.1097/00007890-200204150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Higashi H., Takenaka K., Fukuzawa K., Yoshida Y., Sugimachi K. Restoration of Atp Contents in the Transplanted Liver Closely Relates to Graft Viability in Dogs. Eur. Surg. Res. 1989;21:76–82. doi: 10.1159/000129006. [DOI] [PubMed] [Google Scholar]
- 26.Coombes J.S., Powers S.K., Demirel H.A., Jessup J., Vincent H.K., Hamilton K.L., Naito H., Shanely R.A., Sen C.K., Packer L., Ji L.L. Effect of combined supplementation with vitamin E and alpha-lipoic acid on myocardial performance during in vivo ischaemia-reperfusion. Acta Physiol. Scand. 2000;69:261–269. doi: 10.1046/j.1365-201x.2000.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Young A.J., Lowe G.M. Antioxidant and prooxidant properties of carotenoids. Archives Biochem. Bioph. 2001;5:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 28.Palozza P., Luberto C., Calviello G., Ricci P., Bartoli G.M. Antioxidant and prooxidant role of beta-carotene in murine normal and tumor thymocytes: Effects of oxygen partial pressure. Free Radic. Biol. Med. 1997;22:1065–1073. doi: 10.1016/s0891-5849(96)00498-4. [DOI] [PubMed] [Google Scholar]
- 29.Palozza P., Serini S., Trombino S., Lauriola L., Ranelletti F.O., Calviello G. Dual role of beta-carotene in combination with cigarette smoke aqueous extract on the formation of mutagenic lipid peroxidation products in lung membranes: dependence on pO2. Carcinogenesis. 2006;27:2383–2391. doi: 10.1093/carcin/bgl074. [DOI] [PubMed] [Google Scholar]
- 30.Beatrice M.C., Stiers D.L., Pfeiffer D.R. The Role of Glutathione in the Retention of Ca-2+ by Liver-Mitochondria. J. Biol. Chem. 1984;259:1279–1287. [PubMed] [Google Scholar]
- 31.Bradham C.A., Stachlewitz R.F., Gao W.S., Qian T., Jayadev S., Jenkins G., Hannun Y., Lemasters J.J., Thurman R.G., Brenner D.A. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25:1128–1135. doi: 10.1002/hep.510250514. [DOI] [PubMed] [Google Scholar]
- 32.Jaeschke H. Glutathione Disulfide as Index of Oxidant Stress in Rat-Liver During Hypoxia. American Journal of Physiology. 1990;258:G499–G505. doi: 10.1152/ajpgi.1990.258.4.G499. [DOI] [PubMed] [Google Scholar]
- 33.Masella R., Di Benedetto R., Vari R., Filesi C., Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Demir S., Inal-Erden M. Pentoxifylline and N-acetylcysteine in hepatic ischemia/reperfusion injury. Clinica Chimica Acta. 1998;275:127–135. doi: 10.1016/s0009-8981(98)00078-3. [DOI] [PubMed] [Google Scholar]
- 35.Richman P.G., Meister A. Regulation of Gamma-Glutamyl-Cysteine Synthetase by Non-Allosteric Feedback Inhibition by Glutathione. J. Biol. Chem. 1975;250:1422–1426. [PubMed] [Google Scholar]