Abstract
Effects of azuki bean juice supplementation, prescribed according to a Kanpo medicine regimen, on serum lipid concentrations were studied. Healthy young Japanese women were recruited and were randomly assigned to one of the three groups using a parallel-group design. Control (n = 10), azuki (n = 11) and Concentrated azuki (CA) (n = 12) juice groups consumed 150 g daily of the isocaloric assigned juice for one menstrual cycle with their usual diet. Triglyceride concentrations were decreased in the azuki juice group (p<0.05) and tended to be decreased in the CA juice group (p = 0.055). Triglyceride concentrations in the azuki and CA juice groups decreased by 0.170 mmol/liter (15.4%) and 0.159 mmol/liter (17.9%), respectively (p<0.05). The azuki and CA juice used in this study inhibited pancreatic lipase activity 29.2% and 56.9%, respectively, in vitro. Lipid peroxide changes, based on ANCOVA with the initial level and α-tocopherol changes as covariates, did not differ among the three groups. Serum low density lipoprotein-cholesterol and high density lipoprotein-cholesterol (HDL) cholesterol concentrations did not change. Thus, azuki bean juice intake, as a traditional Kampo prescription, might be beneficial for preventing hypertriglyceridemia.
Keywords: azuki, triglyceride, polyphenol, pancreatic lipase
Introduction
Azuki beans (Phaseolus angularis WIGHT.) have long been widely cultivated and consumed in confectionary and other traditional dishes, in Asian countries. Shozu-to is a Chinese medicine, composed mainly of azuki beans, which has been recognized to have antidotal, diuretic and laxative effects and is thus used to treat constipation, beriberi, nephritis, and insufficient postpartum lactation [1]. Boiled azuki bean juice prescribed by herbal doctors and as a folk remedy has been used to prevent damage associated with the stress of aging. However, the mechanisms underlying these properties have yet to be clarified.
Recently, considerable interest has focused on plant polyphenols because of their antioxidant, anti-inflammatory and anti-proliferative activities. The use of polyphenols as therapeutic agents for cardiovascular disease and stroke has been suggested [2–5]. As part of the strategy to reduce the risk of atherogenic disease, triacyglycerol (TG) reduction with specific foods and dietary modifications is also important for preventing metabolic syndrome [6–8]. Azuki beans reportedly contain polyphenols rich in proanthocyanidin, as well as other catechins and chlorogenic acid [9, 10]. Azuki bean extracts have an inhibitory effect on malonaldehyde formation, and thereby exert antioxidant activity [11–13]. Azuki beans have also been suggested to have lipase inhibitory activity in vitro [14, 15]. These results raise the possibility that azuki beans may decrease serum TG concentrations.
Many therapeutic agents for hyperlipidemia are available, but the mild effects of traditional Kampo medicine could be useful for early prevention. No study has yet focused on the relationship between azuki bean extract intake and serum lipid concentrations in humans, but the little evidence available suggests that ingesting boiled azuki bean juice may reduce the risk of cardiovascular disease. In this study, the lipase inhibitory activities of azuki bean juice were confirmed, and the effects of azuki bean juice supplementation on serum lipid concentrations and oxidative stress marker were investigated in young Japanese women.
Subjects and Methods
Subjects
Healthy women’s student volunteers, with regular menstrual cycles, were recruited through leaflet distribution within our university. A total of 39 students applied for enrollment in this study. None had previously consumed azuki bean juice. Exclusion criteria were; smoking, any current metabolic disease, screening detection with a purchased kit (Phenotyping ApoE, Jyoko Co. Ltd. Japan) of an apoE phenotype other than 2/3, 3/3 or 4/3, regular use of prescription medications, use of vitamins and/or supplements, habitual strenuous exercise, alcohol consumption; and dietary habits not representative of the general population.
Study protocol
We performed a randomized double-blind parallel-group trial. The study protocol was approved by the Tokyo Medical University Institutional Review Board and written informed consent was obtained from all participants.
The subjects were randomly divided into three groups; control, azuki and concentrated azuki (CA) juice. All drank 150 g of the isocaloric assigned juice 5 times in a day for one menstrual cycle, along with consumption of their usual diet. The subjects were instructed to drink 30 g of the assigned juice with each meal and between meals (5 times in total) according to the Kampo prescription, and to record how they consumed the experimental juices on a daily basis. All subjects were instructed to not change their dietary habits or consume special foods during the study period. In particular, soybean and soybean products, fruits, vegetables, coffee and tea consumptions were kept constant and vitamin and nutritional supplement use were strictly avoided. They were also asked to engage in their usual daily activities, but without travel or intense exercise. To evaluate adherence to the required diet, habitual foods, beverages, alcohol, vitamin and supplement uses were recorded daily. To assess physical condition, their bowel movements, medications and physical activities were also recorded daily.
Dietary records were kept for 3 days each prior to and after completion of the study, and were confirmed by an interview with a dietitian. Nutrient intakes were calculated using Wellness/Win (Top Business System Co. Ltd., Okayama) software based on the table of nutrient contents published by the Japanese Science and Technology Agency. Thiamin, riboflavin and niacin intakes, which affect energy metabolism, were calculated per 1000 kcal (=4,184 kJ) of energy consumed. Other results are shown as per standard body weight, based on a body mass index = 22. At baseline and at the end of the experiment, height and weight were measured again.
Two of the subjects took medicines for colds during the experimental period, two showed compliance with the juice consumption below 80%, and two had irregular menstruation. Thus, 33 subjects in total, i.e. 10 controls, 11 azuki juice and 12 CA juice, had evaluable data in this study.
Experimental juice preparation
The azuki juice was made according to the Kampo medicine decoction. Thirty-five g/person/day of azuki beans (Erimosekishozu) were slowly boiled down with 280 g of distilled water for 40 minutes in an aluminum pan. The stock was filtered through strainer and a 150 g clear layer was obtained, i.e. CA juice. This concentrate was diluted to obtain azuki juice of the same volume as the boiled distilled water. Control juice was made with 0.75 g of sucrose in 150 g of boiled distilled water. The experimental juice was made in our laboratory and 150 g were stored in plastic bottles at −20°C. Each subject received her juice for the following week, every weekend, and stored it at −20°C, in a home refrigerator. The nutrient contents of the experimental juices were measured by Japan Food Research Laboratories, Tokyo. The CA juice contained 1.05 g and 0.15 g carbohydrate and protein, respectively, but no dietary fiber per 150 g. The azuki juice contained those nutrients half as the CA juice. Total energy of 4.5 kcal (=18.75 kJ) was provided from 150 g of each experimental juice for a day. The azuki and CA juice contained total polyphenol 880 and 1960 mg, respectively, per 150 g measured by the method of George et al. [16].
Blood sampling and analysis
Blood lipid levels are recognized to differ between the follicular and luteal phases [17, 18]. Oral temperatures were measured every morning to identify the most appropriate day for blood collection during the luteal phase of the menstrual cycle. After oral temperatures rose during the ovulatory phase, the 11th or 12th day with a high oral temperature was taken as the day for blood collection at baseline. The same procedure was followed for the blood sample taken upon completion of the experiment. Blood samples were obtained after a 12-h overnight fast, for measurement of serum lipid and biochemical parameter concentrations. Serum samples were allowed to clot at room temperature and then centrifuged at 1000 × g for 10 min at 15°C.
Serum lipid and oxidation parameter analysis
Total serum cholesterol, high density lipoprotein-cholesterol (HDL-C), TG levels were measured enzymatically. HDL-C was measured after precipitation of very low density lipoprotein and low density lipoprotein (LDL) in the infranatant with heparin and MnCl2. LDL-cholesterol was measured enzymatically. Serum aspartate aminotransferase (AST), alanine aminotransferase, and gamma-glutamyltransferase were measured by the UV, and L-γ-glutamyl-3-carboxy-4-nitroanilide substrate methods, respectively. These biochemical parameters were measured in the laboratory of SRL Co. Ltd, Tokyo. The levels of serum thiobarbitulic acid reactive substances (TBARS) as indices of the products of lipid peroxidation were measured [19].
Serum α-tocopherol content
Serum α-tocopherol was measured using a modification of the Abe and Katsui method , with high performance liquid cromatography [20]. α-Tocopherol was extracted from 250 µl of serum with 2.5 ml of n-hexane after precipitating the proteins with ethanol. Two milliliters of n-hexane extract were evaporated under nitrogen gas, and the residue was dissolved in 50 µL of 2,2,5,7,8-pentametyl-6-hydroxychroman (PMC) (1.5 µg/ml) in hexane. The chromatographic conditions used with the fluorometric detector, Shimadzu LC6A, were as follows: column, Shim-pack CLC-NH (150 × 4.6 mm) (Shimadzu, Co., Japan), mobile phase, isopropyl alcohol/n-hexane (2.8:100, v:v); flow rate, 1.5 ml/min; and injection volume, 10 µl. Results were accepted as valid if the CV for a control sample of a known concentration was below 5%.
Lipase inhibitory activity measurement
The CA juice was diluted to various levels relative to the control. The activity of pancreatic lipase was measured according to the method of Han et al. [21]. An emulsion (9 mL) containing 80 mg of trioleoylglycerol, 10 mg phosphatidylcholine, and 5 mg sodium taurocholate in 0.1 mol/liter N-tris(hydroxymethyl)methyl-2-aminoethane sulfonic acid (TES) buffer (pH 7.0) containing 0.1 mol/liter sodium chloride was prepared by sonication and kept at 37°C. A total of 100 µl of the emulsion was incubated with 5 U of porcine pancreatic lipase (Sigma) solubilized in 0.1 mol/liter TES buffer containing 0.1 mol/liter sodium chloride and various amounts of CA juice (every 10 µl from 0 to 100 µl of CA juice filled up to total 100 µl with distilled water as each sample) at 37°C for 30 min. Released fatty acids were extracted with chloroform:heptane:methanol (49:49:2, v:v:v) and colorimetrically measured. The lipase activity at azuki juice free was expressed as 100%.
Statistical analysis
The data were analyzed using SPSS software (version 12.0) (SPSS Inc. Japan). Data are expressed as means ± SD; values of p<0.05 were considered significant. Percentages of the apoE phenotype were compared among the 3 groups using the chi-square test. Normality was assessed by the Kolmogorov-Smirnov test. Between-group comparisons at baseline were assessed using the Mann-Whitney U test. Changes in each parameter from baseline values were compared using Wilcoxon’s signed rank test. To minimize the potential influence of differences in baseline and other independent values, analysis of covariance was used. Serum lipid and biochemical parameter changes were analyzed, with the baseline data as covariates. Lipid peroxide changes were analyzed, with the baseline data and α-tocopherol changes as covariates. The significance of individual differences among the 3 groups was evaluated using the Fisher LSD post hoc test.
Results
Baseline characteristics of subjects, changes in body mass index (BMI) and consumption state during the study
The mean age of the 33 subjects was 21.3 ± 0.8 years and BMI was 19.8 ± 1.5. There were no significant differences in BMI or apoE phenotype frequency among the three groups. Compliance with the experimental juice consumptions during the study was 94.1 ± 6.7% for all enrolled subjects, and did not differ among the groups. Body weight increased slightly in the controls at the end of the study period (p<0.05) (Table 1). Based on the daily physical condition records, no subjects experienced diarrhea or malaise during this study.
Table 1.
Baseline characteristics of subjects, changes in BMI and consumption state during the study
| Control juice | Azuki juice | Concentrated Azuki juice | ||
|---|---|---|---|---|
| ApoE phenotype | ||||
| 2/3 / 3/3 / 3/4 | 2/7/1 | 2/7/2 | 0/8/3 | |
| Age (year) | 21.2 ± 1.01 | 21.5 ± 0.5 | 21.3 ± 0.7 | |
| Height (cm) | 160.5 ± 3.6 | 158.7 ± 4.2 | 159.9 ± 4.9 | |
| Weight (kg) | baseline | 50.5 ± 2.6 | 50.3 ± 5.7 | 50.9 ± 5.4 |
| end | 51.1 ± 3.0* | 50.3 ± 5.8 | 50.8 ± 5.5 | |
| Consumption (days) | 25.1 ± 3.1 | 27.5 ± 2.7 | 26.8 ± 2.4 | |
| Compliance to cosume (%) | 92 ± 9 | 93 ± 7 | 97 ± 3 | |
| Volume consumed (ml/d) | 138 ± 13 | 140 ± 10 | 145 ± 4 |
Control juice group (n = 10), Azuki juice group (n = 11), Concentrated Azuki juice group (n = 12)
1 Values are means ± SD
*: Significant difference between baseline and final values at p<0.05.
Nutritent intake
In all subjects, energy intakes per standard body weight was 30.7 ± 5.5 kcal (128 ± 23 kJ) and the percentage of energy derived from fat was close to 30. The ratio of saturated fatty acids (SFA):monounsaturated fatty acids: polyunsaturated fatty acids (PUFA) was 1:1.04:0.70, and the (n-6)-PUFA:(n-3)-PUFA ratio was 4:1 at baseline. There were no significant differences in dietary intake at baseline among the three groups. During the intervention period, no significant changes in dietary intake were observed (Table 2).
Table 2.
Energy and nutrient intakes at baseline
| Energy (kcal/kg)1 | 30.7 ± 5.52 |
| (kJ/kg) | 128 ± 232 |
| Protein (g/kg) | 1.11 ± 0.23 |
| Fat, energy% | 30.4 ± 5.7 |
| Thiamin (mg/1000 kcal) | 0.50 ± 0.10 |
| Riboflavin (mg/1000 kcal) | 0.72 ± 0.21 |
| Niacin (mg/1000 kcal) | 7.8 ± 2.3 |
| Fiber (g/kg) | 0.23 ± 0.08 |
| Cholesterol (mg/kg) | 4.8 ± 2.2 |
| SFA (mg/kg) | 268 ± 119 |
| MUFA (mg/kg) | 276 ± 97 |
| n-6 PUFA (mg/kg) | 150 ± 52 |
| n-3 PUFA (mg/kg) | 37 ± 13 |
| Carotene (µg/kg) | 56 ± 33 |
| Ascorbic acid (mg/kg) | 2.1 ± 1.7 |
| α-Tocopherol (mg/kg) | 0.13 ± 0.11 |
n = 33
1 Values are expressed per standard body weight
2 Values are means ± SD
Serum lipid and biochemical parameter concentrations
At baseline, the α-tocopherol concentration was lower in the azuki juice group than in the control juice group and AST was higher in the CA juice group (p<0.05).
At the end of the experiment period, TG was decreased in the azuki juice group (p<0.05) and tended to decrease in the CA juice group (p = 0.055). TC and α-tocopherol concentrations were increased in the controls (p<0.05) (Table 3).
Table 3.
Serum lipid and biochemical parameter concentration changes with consumption of experimental juices
| Control juice | Azuki juice | Concentrated Azuki juice | |||
|---|---|---|---|---|---|
| Total cholesterol | (mmol/liter) | baseline | 4.47 ± 0.621 | 4.81 ± 0.39 | 4.75 ± 0.51 |
| end | 4.83 ± 0.67# | 4.71 ± 0.61 | 4.73 ± 0.66 | ||
| LDL-cholesterol | (mmol/liter) | baseline | 2.34 ± 0.52 | 2.59 ± 0.40 | 2.60 ± 0.50 |
| end | 2.49 ± 0.53 | 2.54 ± 0.63 | 2.56 ± 0.57 | ||
| HDL-cholesterol | (mmol/liter) | baseline | 1.89 ± 0.42 | 1.85 ± 0.32 | 1.82 ± 0.25 |
| end | 2.01 ± 0.50 | 1.83 ± 0.20 | 1.85 ± 0.24 | ||
| Triacylglycerol | (mmol/liter) | baseline | 0.73 ± 0.38 | 0.75 ± 0.35 | 0.64 ± 0.18 |
| end | 0.75 ± 0.31 | 0.61 ± 0.29# | 0.53 ± 0.17 | ||
| Aspartate aminotransferase | (µkat/liter) | baseline | 0.27 ± 0.05 | 0.31 ± 0.05 | 0.32 ± 0.06* |
| end | 0.30 ± 0.09 | 0.32 ± 0.07 | 0.32 ± 0.04 | ||
| Alanine aminotransferase | (µkat/liter) | baseline | 0.24 ± 0.09 | 0.24 ± 0.08 | 0.22 ± 0.07 |
| end | 0.27 ± 0.15 | 0.30 ± 0.12 | 0.19 ± 0.04 | ||
| Gamma- glutamyltransferase | (µkat/liter) | baseline | 0.28 ± 0.06 | 0.24 ± 0.07 | 0.25 ± 0.07 |
| end | 0.29 ± 0.10 | 0.23 ± 0.07 | 0.24 ± 0.05 | ||
| α-tocopherol | (µmol/liter) | baseline | 42.0 ± 8.4 | 34.6 ± 6.3* | 43.2 ± 3.9 |
| end | 47.1 ± 10.9# | 33.4 ± 6.0 | 42.7 ± 4.9 | ||
| Lipid peroxide | (nmol/ml) | baseline | 2.20 ± 0.16 | 2.40 ± 0.20 | 2.50 ± 0.24 |
| end | 2.30 ± 0.21 | 2.40 ± 0.22 | 2.50 ± 0.22 |
Control juice group (n = 10), Azuki juice group (n = 11), Concentrated Azuki juice group (n = 12)
1 Values are means ± SD
*: Significant difference vs Controls at p<0.05.
#: Significant change from baseline value at p<0.05.
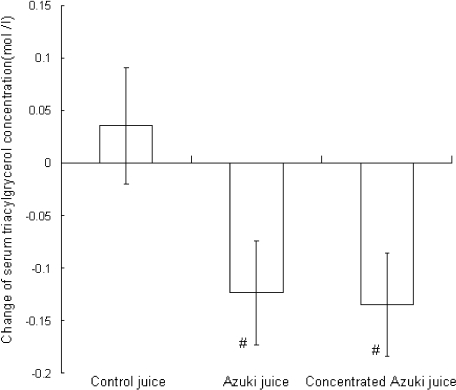
Reductions in TG concentrations in the azuki and CA juice groups were 0.170 mmol/liter (15.4%) and 0.159 mmol/liter (17.9%), respectively (p<0.05), those were different from control based on ANCOVA adjusted for baseline values of TG and AST (p<0.05) (Fig. 1). Lipid peroxide changes based on analysis of covariance, with the initial level and α-tocopherol change as covariates, did not differ among the three groups.
Fig. 1.
Changes in serum triacylglycerol concentration in control (n = 10), azuki (n = 11) and concentrated azuki (n = 12) juice group during the study. Values are presented as means ± SE. # Significantly different from controls at p<0.05 based on ANCOVA adjusted for baseline values of triacyglycerol and aspartate aminotransferase.
Pancreatic lipase activity in vitro
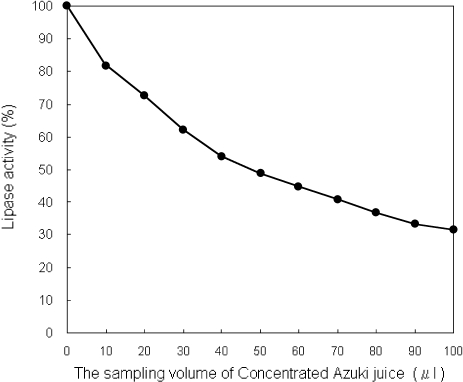
Pancreatic lipase activity was dose-dependently inhibited by the addition of CA juice. Relative to the value of azuki bean juice free control, the azuki bean (as the value of 50 µl) and CA juices (as the value of 100 µl) used in this study decreased pancreatic lipase activity to 51.7% and 32.6%, respectively, in vitro (Fig. 2).
Fig. 2.
Pancreatic lipase activity. The lipase activity at azuki juice free was expressed as 100%. The sample volume of 50 and 100 µl are equivalent to azuki and concentrated azuki bean juice, respectively.
Discussion
Shozu-to, a Chinese medicine composed of azuki beans, has been reported to have various effects and is used to treat constipation, beriberi, nephritis, and inadequate postpartum lactation. However, no report has focused on blood lipid concentrations in humans. The effects of polyphenols on lipid metabolism are rather limited in vivo, as compared with the effects in vitro [22]. In this study the young healthy subjects showed 15.4% and 17.9% of significant TG reductions, in the azuki and CA juice groups, respectively. The clinical significant effects of TG reduction on eicosapentaenoic acid in normolipidemic subjects were considered to exceed 20% [23]. Our result was thought to be a milder effect than that of eicosapentaenoic acid.
In both azuki juice groups, energy, carbohydrate and (n-3)-PUFA intakes, which are considered to affect the serum TG level, remained stable during the experimental period. Furthermore, no changes in dietary habits or physical activities were observed based on daily records. In the control group, there was a slight weight gain despite isocaloric drinks having been provided. The considerable reason for the body weight increase in the control group might be the physiological adaptation of seasonal change for body fat storage in autumn [24]. We performed the study from September to December when the mean temperature decreased from 24°C to 13°C. However, body weight changes did not correlate with TG changes in any of the three groups.
The effects of flavonoid supplementation on TG reduction have already been reported. The tea catechins reportedly have dose-dependent inhibitory effects on pancreatic lipase activity in vitro, thereby suppressing postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats [25]. In humans, tea catechin extracts have also been reported to reduce postprandial TG [26]. One of the major mechanisms decreasing TG is inhibition of intestinal lipid absorption based on the inhibitory effects of catechins on gastric and pancreatic lipases [25, 27]. However, green tea consumption was epidemiologically demonstrated to not be associated with serum TG levels [28], and there have been no reports on the effects of routinely prepared green tea on serum lipids [8]. The azuki and CA bean juices used in this study also decreased pancreatic lipase activity in vitro. The intensity of the inhibitory effect was almost the same as that of green tea routinely prepared for drinking in Japan in vitro. From the dietary records, the subjects kept to consume green tea 480 ± 890 ml/day and 450 ± 450 ml/day at the baseline and the end of the experiment, respectively, and no difference were observed among the groups. There were no correlation between the volume of tea consumption and serum TG level.
Supplemented volume of experimental azuki juice was only 150 ml, about one third of habitual tea consumption. One of the main polyphenols identified in azuki beans is proanthocyanidin [9]. Thus, the significant TG reduction without change in HDL and LDL cholesterol obtained with the prescribed traditional azuki juice was suspected to mainly be attributable to the inhibitory effect on lipase activity. There has been concern that CA juice might have unwanted laxative effects. However, none of our subjects experienced diarrhea or malaise during this study. Lipid excretion into feces should be measured to assess the effects of increased lipase inhibitory activity in the intestine, and further study is needed to evaluate in vivo and the clinical significance of the effect in hypertriglyceridemic patients.
In human studies, anthocyanins were found to be directly absorbed but in small amounts [29, 30]. Further investigation is needed to determine whether azuki juice polyphenols suppress fatty acid synthesis and those lipase functions.
Recently, there has been considerable interest in plant polyphenols such from blueberries [31] and lyophilized grape powder [32], because of their antioxidant activities [33]. Remarkable radical scavenging activities of azuki bean proanthocyanidins have been demonstrated in vitro [9, 10]. Lee and others reported that azuki bean aroma extracts exert their antioxidant activity via an inhibitory effect on malonaldehyde formation [11, 12], comparable to that of α-tocopherol. In our present human experiment, changes in the lipid peroxide levels as measured by TBARS did not differ among the groups. A major reason for this finding is that our subjects were healthy young women, whose serum lipid levels were apparently lower than those of the earlier study subjects described above. The second major reason is that we obtained the serum samples after an overnight fast, although the half–life of plasma total anthocyanin is reportedly 132 min after consumption [34]. We need to examine the postprandial condition to observe the actual oxidative stress state. α-Tocopherol is recognized as a strong antioxidant in the fat-soluble phase. In our subjects, other antioxidants might not have affected the lipid peroxide level, because the serum α-tocopherol concentration was not particularly high, and the volumes of α-tocopherol and ascorbic acid consumed were lower than the recommended adequate intakes for Japanese. Further studies are needed to clarify the anti-oxidative effect of azuki juice consumption in patients with hypertriglyceridemia susceptible to oxidative stress.
In conclusion, the TG concentration decreased with consumption of azuki bean juice. The mechanism is suspected to involve mainly suppression of lipase inhibitory activity. Azuki bean juice intake as a traditional Kampo prescription might be beneficial for preventing hypertriglyceridemia.
Acknowledgement
We deeply appreciate Dr. Terashi for his advice on Kanpo medicine decoction.
References
- 1.Namba T. In: Phaseoli Semen, in A full-color illustrated book of Japanese Flora. Namba T., editor. Hoikusha.; Tokyo: 1980. p. 298. [Google Scholar]
- 2.Kris-Etherton P.M., Hecker K.D., Bonanome A., Coval S.M., Binkoski A.E., Hilpert K.F., Griel A.E., Etherton T.D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002;113 9B:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 3.Simonyi A., Wang Q., Miller R.L., Yusof M., Shelat P.B., Sun A.Y., Sun G.Y. Polyphenols in cerebral ischemia novel targets for Neuroprotection. Mol. Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- 4.Vita J.A. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005;81 Suppl:292S–297S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 5.Arts I.C.W., Hollman P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81 Suppl:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization, author. World Health organization.; Switzerland: 1999. Definition, diagnosis and classification of diabetes and its complications: report of a WHO Consultation. Part 1: diagnosis and classification of diabetes mellitus. [Google Scholar]
- 7.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NECP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 8.Iso H., Naito Y., Sato S., Kitamura A., Okamura T., Sankai T., Shimamoto T., Iida M., Komachi Y. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am. J. Epidemiol. 2001;153:490–499. doi: 10.1093/aje/153.5.490. [DOI] [PubMed] [Google Scholar]
- 9.Ariga T., Koshiyama I., Fukushima D. Antioxidative properties of procyanidins B-1 and B-3 from azuki beans in aqueous system. Agric. Biol. Chem. 1988;52:2717–2722. [Google Scholar]
- 10.Ariga T., Hamano M. Radical scavenging action and its mode in procyanidins B-1, and B-3 from azuki beans to peroxyl radicals. Agric. Biol. Chem. 1990;54:2499–2504. [Google Scholar]
- 11.Lee K.G., Mitchell A. E., Shibamoto T. Determination of antioxidant properties of aroma extracts from various beans. J. Agric. Food Chem. 2000;48:4817–4820. doi: 10.1021/jf000237e. [DOI] [PubMed] [Google Scholar]
- 12.Lee K.G., Shibamoto T., Takeoka G.R., Lee S.E., Kim J.H., Park B.S. Inhibitory effects of plant-derived flavonoids and phenolic acids on malonaldehyde formation from ethyl arachidonate. J. Agric. Food Chem. 2003;51:7203–7207. doi: 10.1021/jf0345447. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.J., Lee K.G. . Inhibitory effects of volatile antioxidants found in various beans on malonaldehyde formation in horse blood plasma. Food Chem. Toxicol. 2005;43:515–520. doi: 10.1016/j.fct.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Shimura S., Itoh Y., Yamashita A., Kitano A., Hatano T., Yoshida T., Okuda T. Inhibitory effects of flavonoids on lipase. Nippon Shokuhin Kogyo Gakkaishi. 1994;41:847–850. [Google Scholar]
- 15.Hatano T., Yamashita A., Hashimoto T., Ito H., Kubo N., Yoshiyama M., Shimura S., Itoh Y., Okuda T., Yoshida T. Flavan dimers with lipase inhibitory activity from Cassia Nomame. Phytochemistry. 1997;46:893–900. [Google Scholar]
- 16.Gerorgé S., Brat P., Alter P., Amiot M.J. Rapid determination of polyphenols and vitamin C in plant derived products. J. Agric. Food Chem. 2005;53:1370–1373. doi: 10.1021/jf048396b. [DOI] [PubMed] [Google Scholar]
- 17.Muesing R.A., Forman M.R., Graubard B.I., Beecher G.R., Lanza E., McAdam P.A., Campbell W.S., Olson B.R. Cyclic changes in lipoprotein and apolipoprotein levels during the menstrual cycle in healthy premenopausal women on a controlled diet. J. Clin. Endocrinol. Metab. 1996;81:3599–3603. doi: 10.1210/jcem.81.10.8855808. [DOI] [PubMed] [Google Scholar]
- 18.Barnett J.B., Woods M.N., Lamon-Fava S., Schaefer E.J., McNamara J.R., Spiegelman D., Hertzmark E., Goldin B., Longcope C., Gorbach S.L. Plasma lipid and lipoprotein levels during the follicular and luteal phases of the menstrual cycle. J. Clin. Endocrinol. Metab. 2004;89:776–782. doi: 10.1210/jc.2003-030506. [DOI] [PubMed] [Google Scholar]
- 19.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 20.Abe K., Katsui G. Determination of tocopherols in serum by high-speed liquid chromatography. Vitamins. 1975;49:259–263. doi: 10.3177/jnsv.21.183. [DOI] [PubMed] [Google Scholar]
- 21.Han L.K., Xu B.J., Kimura Y., Zheng Y.N., Okuda H. Platycodi radix affects lipid metabolism in mice with high fat diet-induced obesity. J. Nutr. 2000;130:2760–2764. doi: 10.1093/jn/130.11.2760. [DOI] [PubMed] [Google Scholar]
- 22.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81 Suppl.:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 23.Harris W.S. Fish oils and plasma lipid and lipoprotein metabolism in humans; A critical review. J. Lipid. Res. 1989;30:785–807. [PubMed] [Google Scholar]
- 24.Umemiya N. Seasonal variations of physiological characteristics and thermal sensation under identical thermal conditions. J. Physiol. Anthropol. 2006;25:29–39. doi: 10.2114/jpa2.25.29. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda I., Tsuda K., Suzuki Y., Kobayashi M., Unno T., Tomoyori H., Goto H., Kawata Y., Imaizumi K., Nozawa A., Kakuda T. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J. Nutr. 2005;135:155–159. doi: 10.1093/jn/135.2.155. [DOI] [PubMed] [Google Scholar]
- 26.Unno T., Tago M., Suzuki Y., Nozawa A., Sagesaka Y.M., Kakuda T., Egawa K., Kondo K. Effect of tea catechins on postprandial plasma lipid responses in human subjects. Br. J. Nutr. 2005;93:543–547. doi: 10.1079/bjn20041379. [DOI] [PubMed] [Google Scholar]
- 27.Juhel C., Armand M., Pafumi Y., Rosier C., Vandermander J., Lairon D. Green tea extract (AR25) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J. Nutr. Biochem. 2000;11:45–51. doi: 10.1016/s0955-2863(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 28.Tokunaga S., White I.R., Frost C., Tanaka K., Kono S., Tokudome S., Akamatsu T., Moriyama T., Zakouji H. Green tea consumption and serum lipids and lipoproteins in a population of healthy workers in Japan. Ann. Epidemiol. 2002;12:157–165. doi: 10.1016/s1047-2797(01)00307-6. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto H., Inaba H., Kishi M., Tominaga S., Hirayama M., Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001;49:1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- 30.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81 Suppl.:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 31.Mazza G., Kay C.D., Cottrell T., Holub B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002;50:7731–7737. doi: 10.1021/jf020690l. [DOI] [PubMed] [Google Scholar]
- 32.Zern T.L., Wood R.J., Greene C., West K.L., Liu Y., Aggarwal D., Shachter N.S., Fernandez M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005;135:1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 33.Lila M.A. Anthocyanins and human health: an in vitro investigative approach. J. Biomed. Biotechnol. 2004;5:306–313. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao G., Muccitelli H.U., Sanchez-Moreno. C., Prior R.L. Anthocyanins are absorbed in glycated forms in elderly women: a pharmacokinetic study. Am. J. Clin. Nutr. 2001;73:920–926. doi: 10.1093/ajcn/73.5.920. [DOI] [PubMed] [Google Scholar]