Abstract
Since we have been exposed to excessive amounts of stressors, aromatherapy for the relaxation has recently become very popular recently. However, there is a problem which responds to light with the essential oil used by aromatherapy. It is generally believed that singlet oxygen is implicated in the pathogenesis of various diseases such as light-induced skin disorders and inflammatory responses. Here we studied whether essential oils can effectively scavenge singlet oxygen upon irradiation, using the electron spin resonance (ESR) method. Green light was used to irradiate twelve essential oils from rutaceae. Among these twelve essential oils, eight were prepared by the expression (or the compression) method (referred to as E oil), and four samples were prepared by the steam distillation method (referred to as SD oil). Five E oils enhanced singlet oxygen production. As these essential oils may be phototoxic, it should be used for their use whit light. Two E oils and three SD oils showed singlet oxygen scavenging activity. These results may suggest that the antioxidant activity of essential oils are judged from their radical scavenging activity. Essential oils, which enhance the singlet oxygen production and show higher cytotoxicity, may contain much of limonene. These results suggest that limonene is involved not only in the enhancement of singlet oxygen production but also in the expression of cytotoxic activity, and that attention has to be necessary for use of blended essential oils.
Keywords: aromatherapy, essential oil, rutaceae, ESR, singlet oxygen
Introduction
We have recently showed the antioxidant action of twenty eight essential oils that are used frequently in aromatherapy and their influence on the normal dermal fibroblast [1, 2]. In aromatherapy, it is said that essential oils are effective and are applied on the skin for percutaneous absorption [3]. For that reason the use of blended essential oils (with 2–4 different oils) has been popular, as compared with the single use of each essential oil. Essential oils of rutaceae are generally favored for their fragrance and they are used for adjustments of the fragrance in blended essential oils. There are some, but not been scientifically substantiated claims that these oils may exhibit phototoxicity. Despite the frequent use of Orange sweet and Mandarin rutaceae essential oils, their phototoxicity has not been fully characterized so far. It has been reported that nonsteroidal anti-inflammatory drugs such as oxicams is photosensitized by ultraviolet rays, possibly via production of singlet oxygen [4].
Atmospheric oxygen (3O2: triplet oxygen) is a stable form of oxygen. On the other hand, singlet oxygen (1O2) [5] is an active, high-energy form of oxygen and known as one of the reactive oxygen species (ROS) produced in the skin, upon exposure to ultraviolet rays [6]. Singlet oxygen attacks the cells, inducing the hyperoxidation, the oxygen cytotoxicity, the decrease in the antioxidative activity and the light-induced skin damage in the cells [7]. Singlet oxygen may be produced by visible light in the presence of certain photosensitizing dyes [8, 9].
Extract of rutaceae essential oils are prepared from two kinds of method, the expression (or the compression) method (referred to as E oil) and the steam distillation method (referred to as SD oil).
We first studied the possible singlet oxygen scavenging activity of rutaceae essential oils, eight E oils (Bergamot, Grapefruit, Lemon, Lime, Mandarin, Orange sweet, Orange bitter, Yuzu) and four SD oils (Lime, Neroli, Petitgrain, Yuzu), using electron spin resonance (ESR) method. Next, we studied the interaction between singlet oxygen and cytotoxic activity.
Materials and Methods
Materials
The following essential oils and reagents were obtained from the indicated companies: Citrus bergamia (Bergamot), Citrus limon (Lemon), Citrus paradise (Grapefruit), Citrus reticulate (Mandarin), Citrus sinensis (Orange sweet) (purchased SANOFLORE company, France), Citrus aurantium (Orange bitter), Citrus aurantium var. amara (Neroli), Citrus aurantium var. amara (Petitgrain) (PRANAROM company, France), Citrus junos (Yuzu-E oil), Citrus junos (Yuzu-SD oil) (Maji-mura farm co-op, Kochi Japan), Citrus aurantifolia (Lime-E oil) (LIBRA NATUTHERAPY Co., Ltd., Hyogo Japan), Citrus aurantifolia (Lime-SD oil) (GAIA/NP company, Tokyo Japan).
Limonene, Linalool, Rose Bengal, 2,2,6,6-tetramethyl-4-hydroxy-piperidine and Maltol (Wako Pure Chem. Industries Co., Ltd., Osaka, Japan).
Essential oils were dissolved and diluted with ethanol, DMSO (dimethyl sulfoxide) or chloroform (Wako Pure Chem. Industries Co., Ltd., Osaka Japan) for the measurement of cytotoxic activity, singlet oxygen radical measurement and component analysis, respectively.
Essential oils extract methods
Tow kind of methods have been used for extraction for essential oils. The expression method compresses pericarps by machine. The steam distillation method steam flowers, leafs and percarps, oil including aroma components distillation.
Singlet oxygen measurement
Singlet oxygen was generated by a photosensitization reaction with Rose Bengal. Singlet oxygen was indirectly estimated as the peak intensity of 2,2,6,6-tetramethyl-4-hydroxy-piperidinyloxy (4-OH TEMPO) radical produced by the oxidation of 2,2,6,6-tetramethyl-4-hydroxy-piperidine (4-OH TEMP) [10, 11] with singlet oxygen (produced by photosensitization with Rose Bengal), using ESR (JEOL JES, X-band, 100 KHz modulation frequency, JEOL Corporation, Tokyo Japan) [12–15].
Essential oils and composition ingredients (limonene and linalool) were diluted in DMSO to the final concentrations of 0.1, 0.2, 0.4, 0.6, 0.8, 1 and 2%. Limonene did the experiment that added linalool too. Each concentration of limonene (the final concentrations of 0.1, 0.2, 0.4, 0.6, 0.8, 1 and 2%) added the final concentration 0.1% linalool, (and similarly measured singlet oxygen.) Samples( in DMSO, 100 µl), phosphate buffer (pH 7.4 100 µl), l00 mM 4-OH TEMP (40 µl) and 200 µM Rose Bengal (20 µl) were placed in 96 well culture plates (flat-bottom type) by eight and irradiated for 7 min (1.3 J/cm2) by green LED light (LED: Light Emitting Diode Green λ max = 550 nm, Simatec Ltd., Tokyo Japan). After irradiation, plates were kept in dark at room temperature until measurement. Each sample transferred to quartz ESR cell (Radical research Company, Tokyo Japan) for recording the ESR spectrum. The instrument settings were as follows: center field, 335.5 ± 5 mT; microwave power 5 mW; modulation amplitude, 0.1 mT; gain, 2.5 × 100. In case of only DMSO solvent (essential oil 0%) was assigned as 100% singlet oxygen activity, and that is to say 0% radical scavenging activity. Radical scavenging activity was calculated for each essential oil at each concentration.
An absorption maximum wavelength of Rose Bengal is 549 ± 2 nm [16]. Essential oils have absorption in ultraviolet region, but not in a visible region. And so we used the LED right at 550 nm. Maltol (3-hydroxy-2-methyl-4-pyrone) is used as positive control of singlet oxygen scavenging activity [17, 18].
Assay for cytotoxic activity
There were a few studies to use human dermal fibroblast elating to Singlet oxygen [19, 20], and so we experiment to used normal human dermal fibroblast. Normal human dermal fibroblast were cultured in a CS-C culture media kit (Dainippon Sumitomo Pharmaceuticals Co., Ltd., Osaka Japan) supplemented with 10% heat-inactivated FBS under a humidified 5% CO2 atmosphere. The cytotoxic activity was determined with a MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay [21]. Normal human dermal fibroblast were inoculated (1 × 104/100 µl/well) in 96-microwell plate. After incubation for 24 h, the diluted essential oils were added to culture medium to the final concentrations of 0.0025, 0.005, 0.0075, 0.01, 0.025 or 0.05%, and cultured for further 1, 3, 6, 12 or 24 h. Concentration of ethanol used to dilute the essential oils was 0.05%, a concentration that did not affect the cell growth. The relative viable cell number was then determined by a CellTiter 96TM assay (Promega Co., Ltd.,Wisconsis), using a Micro-plate reader (Nippon Bio-Rad Laboratoris Co., Tokyo, Japan). The CellTiter 96TM assay is based on MTT assay and cellular conversion of tetrazolium salt into a formazan product [21].
Component analysis
Essential oils were diluted 1 to 500 in chloroform and injected into the Gas Chromatography-Mass Spectrometry (GC/MS: GC6890, MSD5973, Agilent Techologies, Australia), with helium as carrier gas. Linalool (a monoterpene alcohol) and limonene (a monoterpene hydrocarbon) were chosen as reference ingredients.
Statistical analysis
The signification in the difference between control and treated group was evaluated by Student’s t test. All the data represent the mean values of triplicate measurement.
Results
Singlet oxygen measurement
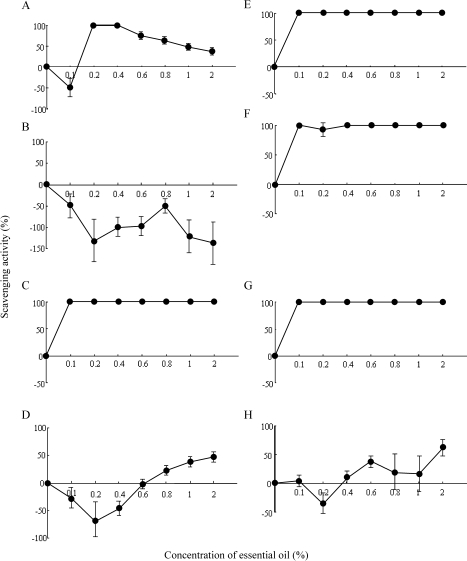
Twelve samples of essential oils were irradiated with green light, and singlet oxygen scavenging activity of these samples was measured by ESR (Table 1). Grapefruit, Lemon, Orange sweet and Orange bitter (all E oils) enhanced the production of singlet oxygen at 0.1–2% (Fig. 1B, Table 1). Neroli (SD oil) showed 100% of singlet oxygen scavenging activity at all concentrations between 0.1 and 2% (Fig. 1E, Table 1). Petitgrain (SD oil) showed 100% of singlet oxygen scavenging activity at 0.1%, 0.4–2% and concentration of 0.2% scavenged 92% (Fig. 1F, Table 1) Lime (E oil and SD oil) also showed a singlet oxygen scavenging activity of 100% at all concentrations between 0.1 and 2% (Fig. 1C, G, Table 1). Bergamot (E oil) completely scavenged the singlet oxygen at 0.2–0.4%. This oil enhanced the singlet oxygen production at 0.1%, whereas it scavenged the singlet oxygen above 0.4% (Fig. 1A, Table 1). Yuzu (E) produced singlet oxygen at 0.1–0.6% and scavenged at 0.8–2% but it scavenged the singlet oxygen by less than 50% (Fig. 1D, Table 1). Yuzu (SD) produced singlet oxygen at 0.2% and scavenged at 0.1%, 0.3–2% but it scavenged the singlet oxygen by less than 50% (Fig. 1H, Table 1).
Table 1.
Rutaceae essential oils required to scavenge in singlet oxygen
| activity | Essential oils |
|---|---|
| scavenging | Lime (E)*, Lime (SD)**, Neroli (SD), Petitagrain (SD) |
| scavenging and enhancement | Bergamot (E), Yuzu (E), Yuzu (SD) |
| enhancement | Grapefruit (E), Lemon (E), Mandarin (E), Orange sweet (E), Orange bitter (E) |
*E: Expression mtehod, **SD: Steam Distillation method
Fig. 1.
Singlet oxygen scavenging activity of retaceae essential oils. A: Bergamot (E) *, B: Grapefruit (E), C: Lime (E), D: Yuzu (E), E: Neroli (SD) **, F: Petitgrain (SD), G: Lime (SD), H: Yuzu (SD) *E: Expression method, **SD: Steam Distillation method The scavenging activity of minus percentages means productions of singlet oxygen. Each value represents the mean and the standard deviation.
Assay for cytotoxic activity
Twelve essential oils were subjected to the MTT assay for cytotoxic activity, using normal human dermal fibroblast as target cells. The 50% cytotoxic concentration (CC50) was determined from the dose-response curve (data not shown) and twelve essential oils were classified three groups by CC50 (Table 2). The results showed that E oils exhibited greater cytotoxic activity than SD oils (Table 2). Enhancement of singlet oxygen production was observed in oils with higher cytotoxic activity (Table 1, 2).
Table 2.
Cytotoxic activity of rutaceae essetial oils
| Concentration of essential oil (%) | Essential oils |
|---|---|
| 0.0075≤CC50*<0.01 (0.0087 ± 0.0013)**** | Bergamot (E)**, Grapefruit (E), Lemon (E), Mandarin (E), Orange sweet (E), Orange bitter (E) |
| 0.01≤CC50<0.025 (0.0203 ± 0.0060) | Yuzu(E), Yuzu(SD)*** |
| 0.025≤CC50<0.05 (0.0313 ± 0.0048) | Lime (E), Lime (SD), Neroli (SD), Petitagrain (SD) |
*CC50: 50% Cytotoxic concentration, **E: Expression mtehod, ***SD: Steam Distillation method, ****: Each value represents the mean and the standard deviation.
Component analysis
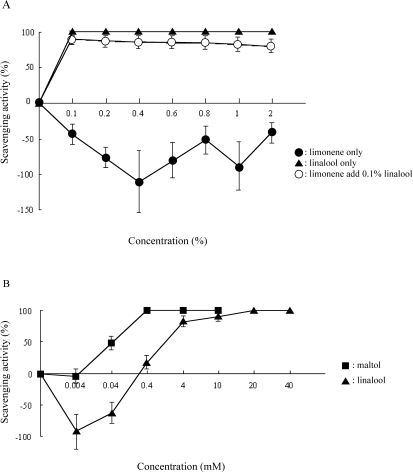
The components of essential oils for limonene and linalool were analyzed (Table 3). Singlet oxygen scavenging activity of limonene, linalool and maltol (positive control) were measured (Fig. 2). Limonene exhibited no scavenging activity over the concentration range of 0.1–2%. Linalool completely scavenged the singlet oxygen at all concentrations between 0.1 and 2%. Though limonene alone has not shown scavenging activity at any concentration, it could scavenge the singlet oxygen radical in combination with final concentration 0.1% linalool (Fig. 2A). Linalool of 0.1% is equivalent to ca. 14 mM.
Table 3.
Component analysis of rutaceae essential oils
| Essential oil | Extraction | Limonene (%) | Linalool (%) | Furocoumarin (%)*** |
|---|---|---|---|---|
| Bergamot | E* | 39.87 | 11.59 | 0.35 |
| Grapefruit | E | 96.35 | ND | 0.01 |
| Lemon | E | 65.46 | 0.08 | 0.01 |
| Mandarin | E | 71.87 | ND | 0.01 |
| Orange (S) | E | 95.95 | 0.27 | ND |
| Orange (B) | E | 96.87 | 0.14 | 0.07 |
| Neroli | SD** | 15.61 | 45.95 | ND |
| Petitgrain | SD | 0.96 | 26.21 | ND |
| Lime (E) | E | 45.06 | 0.08 | 0.25 |
| Lime (SD) | SD | 44.6 | 0.07 | 0.01 |
| Yuzu (E) | E | 79.83 | 0.69 | 0.01 |
| Yuzu (SD) | SD | 78.83 | 1.33 | ND |
Orange(S) is Orange sweet. Orange(B) is Orange bitter.
*E: Expression mtehod, **SD: Steam Distillation method, ***: The data of furocumarin quoted a component analysis list.
ND is less than detection limit.
Fig. 2.
Singlet oxygen scavenging activity of composition ingredients limonene, linalool and maltol. A: Singlet oxygen scavenging activity of limonene and linalool. Closed circle is limonene alone. Closed triangle is linalool alone. Open circle is limonene (the concentration range of 0.1–2%) added final concentration of 0.1% linalool (ca.14 mM). The scavenging activity of minus percentages means productions of singlet oxygen. B: Singlet oxygen scavenging activity of linalool and maltol (as positive control). Closed square is maltol alone ( positive control ). Final concentrations of maltol were 0.004, 0.04, 0.4, 4 or 10 mM. Closed triangle is linalool alone. Final concentrations of linalool were 0.004, 0.04, 0.4, 4, 10, 20 or 40 mM. The scavenging activity of minus percentages means productions of singlet oxygen. Each value represents the mean and the standard deviation.
Discussion
Limonene, a monoterpene hydrocarbon, is a general component of all twelve rutaceae essential oils examined. The oils with strong singlet oxygen scavenging activity have low limonene contents. In contrast, essential oils which enhanced the singlet oxygen production, have high levels of limonene, and low levels of linalool which is a monoterpene alcohol. These results suggest that limonene and linalool are the causative substances for enhancement and scavenging of singlet oxygen, respectively. Singlet oxygen scavenging activity of linalool was demonstrated at very low concentrations, suggesting the possible application of this compound for counteracting with the limonene-stimulated production of singlet oxygen.
Since Bergamot contains lower amounts of limonene as compared with linalool (which is not a component of other the expression method essential oils), it is expected that the increase or decrease of the singlet oxygen scavenging activity may be determined by the ratio of limonene to linalool concentration. In fact it is reported that Bergamot completely scavenged the singlet oxygen at 0.2–0.4%, but it enhanced the singlet oxygen production at 0.1%. However, Bergamot contains bergapten (5-methoxypsoralen: a furocoumarin), that may cause the carcinogenesis as well as phototoxicity under light [22, 23]. Therefore, careful attention is necessary to apply the Bergamot on skin under light.
We found that Lime (SD), Neroli and Petitgrain oils prepared by the steam distillation method showed higher singlet oxygen scavenging activity than those prepared by the expression method, possibly due to the presence of higher linalool and lower limonene levels.
Orange sweet and Mandarin oils have been reported to be a little bit phototoxic, in contrast to our finding that these oils were phototoxic, judging from their singlet oxygen enhancement effect [24–27].
Essential oils prepared by the expression method enhanced the singlet oxygen production and most of them, except for Lime (E oil), were phototoxic. We found that Lime (E oil and SD oil) completely scanvenged the singlet oxygen. Essential Oils that contained bergapten (a furocoumarin) have to be dealt with cautiously due to its phototoxicity. Since Lemon, Orange sweet, Orange bitter and Mandarin oils contain very small amounts of bergapten, it is expected that they may have much lower incidence of phototoxicity and carcinogenesis upon application on skin [22]. Essential oils that produce excessive singlet oxygen and higher cytotoxicity contained higher amounts of limonene. It is suggested that limonene is involved in not only enhancing the singlet oxygen production but also expressing the higher cytotoxicity.
Conclusions
Most of essential oils dose-dependently scavenged singlet oxygen, suggesting their possible antioxidant activities. Several different essential oils are usually blended in Japan. Blending may produce synergistic, antagonistic or yet-unidentified adverse effects by the interaction between hundred of natural organic compounds present in each essential oil sample. Further study of such interaction is necessary for use of essential oils in safe.
References
- 1.Ao Y., Shioda S. Effect of essential oils on survival of normal human dermal fibroblast cells-Evaluation of cytotoxic activity. J. J. S. Aroma. 2006;1:34–40. [Google Scholar]
- 2.Ao Y., Satoh. K ., Sudoh. K ., Shioda S. Anti-oxidation of essential oils—A study of singlet oxygen scavenging activity—. J. J. S. Aroma. 2007;1:55–61. [Google Scholar]
- 3.AEAJ, author. Aroma Environment Association of Japan qualification system committee editing: The first grade aromotherapy official approval text. Tokyo: 2006. pp. 70–71. [Google Scholar]
- 4.Davis P. Aromatherapy, An A-Z. Tokyo: 2004. pp. 293–295. [Google Scholar]
- 5.Satoh K., Sasaki T., Tokuyama S., Yamamoto T., Ida Y. Generation of singlet oxygen on the oxicams in nonsteroidal anti-inflammatory drugs. Mag. Reson. Med. 2001;12:57–60. [Google Scholar]
- 6.Pierlot C., Aubry J.M., Briviba K., Sies H., Di. Mascio P. Naphthalene endoperoxides as generators of singlet oxygen in biological media. Vol. 319. Methods in Enzymology; California: 2000. pp. 3–20. [DOI] [PubMed] [Google Scholar]
- 7.Kochevar E.I., Redmond W.R. Photosensitized production of singlet oxygen. Vol. 319. Methods in Enzymology: California: 2000. pp. 20–28. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa T. Medicine of Free radical. Shindan To Chiryosha, Inc.; Tokyo: 1997. pp. 1–17. (in Japanese) [Google Scholar]
- 9.Di. Mascio P., Devasagayam T.P.A., Kaiser S., Sies H. Cartenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem. Soc. Trans. 1990;18:1054–1056. doi: 10.1042/bst0181054. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi M., Sakamoto Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluyialis. Biotechnol. Lett. 1999;21:265–269. [Google Scholar]
- 11.Jai Ashish Tilak-Jain and Thomas Paul Asir Devasagayam, author. Cardioprotective and Other Beneficial Effects of Some Indian Medicinal Plants. J. Clin. Biochem. Nutr. 2006;38:9–18. [Google Scholar]
- 12.Kon T., Tanigawa T., Hayamizu K., Shen M., Tsuji T., Naito Y., Yoshikawa T. Singlet oxygen quenching activity of human serum. Redox Report. 2004;9:325–330. doi: 10.1179/135100004225006821. [DOI] [PubMed] [Google Scholar]
- 13.Yoshino F., Shoji H., Lee M.C. Vascular effects of singlet oxygen (1O2) generated by photo-exitation on adrenergic neurotransmission in isolated rabbit mesenteric vein. Redox Report. 2002;7:266–270. doi: 10.1179/135100002125000767. [DOI] [PubMed] [Google Scholar]
- 14.Ando T., Yoshikawa T., Tanigawa T., Kohno M., Yoshida N., Kondo M. Quantification of singlet oxygen from hematoporphyrin derivative by electron spin resonance. Life Sci. 1997;61:1953–1959. doi: 10.1016/s0024-3205(97)00835-7. [DOI] [PubMed] [Google Scholar]
- 15.Zang L.Y. van Kujk., Misra B.R., Misra H.P. The specificity and producte of quenching single oxygen by 2,2,6,6-tetramethylpiperidine. Bochem. Mol. Biol. Int. 1995;37:283–293. [PubMed] [Google Scholar]
- 16.Japan Cosmetic Industry Association, author. Handbook of Legal Pigment. Tokyo: 1988. pp. 13–15. [Google Scholar]
- 17.Okuda O. KOURYOUKAGAKUSOURAN 2. Tokyo: 1980. pp. 902–904. [Google Scholar]
- 18.Watanabe-Akanuma M., Inaba Y., Ohta T. Mutagenicity of UV-irradiated maltol in Salmonella typhimurium. Mutagenesis. 2007;22:43–47. doi: 10.1093/mutage/gel057. [DOI] [PubMed] [Google Scholar]
- 19.Tyrrell R.M., Pldoux M. Singlet oxygen involvement in the inactivation of cultured human fibroblasts by UVA (334 nm, 365 nm) and near-visible (405 nm) Photochem. Photobiol. 1989;49:407–412. doi: 10.1111/j.1751-1097.1989.tb09187.x. [DOI] [PubMed] [Google Scholar]
- 20.Scharffetter-Kochanec K., Wlaschek M., Briviba K., Sies H. Singlet oxygen induced collagenase expression in human skin fibroblast. FEBS Lett. 1993;331:304–306. doi: 10.1016/0014-5793(93)80357-z. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immun. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Sue C. Essential Chemistry for Safe Aromatherapy. Tokyo: 2004. pp. 81–82. [Google Scholar]
- 23.Averbeck D., Averbeck S. Genotoxicity of Bergapten and Bergamot oil in Saccharomyces cer evisiae. J. of Photochemistry and Photobiology, B Biology. 1990;7:209–229. doi: 10.1016/1011-1344(90)85158-s. [DOI] [PubMed] [Google Scholar]
- 24.Wanda S. The Directory of Essential Oils. Tokyo: 2005. pp. 12–151. [Google Scholar]
- 25.Kawabata K. Handbook of aromatherapy. Osaka: 2002. pp. 118–173. [Google Scholar]
- 26.Tisserand R., Balacs T. Essential Oils Safety (the second volume) Tokyo: 2005. p. 357. [Google Scholar]
- 27.AEAJ: Aroma Environment Association of Japan qualification system committee editing: The first grade aromotherapy official approval text Tokyo: 22.2006 [Google Scholar]