Abstract
Heparan sulfate proteoglycans bind to and regulate many inflammatory mediators in vitro, suggesting that they serve an important role in influencing inflammatory responses in vivo. Here we evaluated the role of syndecan-1, a major heparan sulfate proteoglycan, in modulating inflammatory responses in Gram-positive toxic shock, a systemic disease that is a significant cause of morbidity and mortality. Syndecan-1-null and wild-type mice were injected intraperitoneally with staphylococcal enterotoxin B, a pyrogenic superantigen, and their inflammatory responses were assessed. Syndecan-1-null mice showed significantly increased liver injury, vascular permeability, and death in response to staphylococcal enterotoxin B challenge compared with wild-type mice. Although serum levels of systemic IL-2 and IFNγ were similar between the two backgrounds, those of TNFα and IL-6 were significantly increased in syndecan-1-null mice undergoing Gram-positive toxic shock. Furthermore, syndecan-1-null mice challenged with staphylococcal enterotoxin B showed enhanced T cell accumulation in tissues, whereas immunodepletion of T cells protected syndecan-1-null mice from the magnified systemic cytokine storm, inflammatory tissue injury, and death. Importantly, syndecan-1 shedding was induced in wild-type mice injected with staphylococcal enterotoxin B, and the administration of heparan sulfate, but not syndecan-1 core protein, rescued syndecan-1-null mice from lethal toxic shock by suppressing the production of TNFα and IL-6, and attenuating inflammatory tissue injury. Altogether, these data suggest that syndecan-1 shedding is a key endogenous mechanism that protects the host from Gram-positive toxic shock by inhibiting the dysregulation and amplification of the inflammatory response.
Gram-positive toxic shock is a systemic inflammatory response syndrome (SIRS)3 where various staphylococcal and streptococcal pyrogenic toxins with superantigenic properties instigate disease, but disease progression is mediated by the dysregulated and exaggerated inflammatory responses of the host. The pyrogenic bacterial toxins function as superantigens by directly binding and activating a large proportion of α/β T cell receptor (TCR)-bearing T cells and MHC class II-positive antigen-presenting cells (APCs) without the need for conventional antigen processing (1–4). For example, in mice, staphylococcal enterotoxin B (SEB) binds to the I-A and I-E MHC class II molecules and to the variable region of certain β chain components (e.g. Vβ3, -7, -8, -17) of the α/β TCR complex, a region not involved in ordinary antigen recognition (2). T cells and APCs activated by pyrogenic superantigens produce large amounts of several pro-inflammatory cytokines. These include TNFα, IL-1β, and IL-6 produced primarily by MHC class II-positive cells, and IL-2, IFNγ, and TNFβ produced by relevant Vβ chain-bearing T cells (1, 3). However, once the initial cytokines are generated, it is likely that they induce additional inflammatory factors, further dysregulating and amplifying the inflammatory response. Furthermore, post-T cell activation mechanisms appear to be critical in Gram-positive toxic shock because although T cells are essential for disease pathogenesis, disease susceptibility is mediated by mechanisms downstream of T cell activation (5). The resultant systemic cytokine storm triggers the clinical manifestations of Gram-positive toxic shock, such as inflammatory tissue damage, vascular leakage, hypotension, disseminated intravascular coagulation, and ultimately lethal shock. Thus, it is important to understand how the expression and activity of superantigen-induced cytokines are regulated in vivo.
Heparan sulfate (HS) and its highly sulfated pharmaceutical analogue, heparin, bind to and regulate many molecules that have been implicated in Gram-positive toxic shock, such as IL-2 (6, 7), IFNγ (8, 9), TNFα (10), and IL-6 (11). Further, several studies have shown that heparin attenuates inflammation in several major inflammatory diseases, such as asthma (12), inflammatory bowel disease (13), and sepsis (14). Although the molecular basis of how heparin inhibits inflammation is not fully understood, its anti-inflammatory activity is not mediated by the anticoagulant domain (12, 15). Native heparin is found in intracellular vesicles of connective tissue mast cells, whereas HS is expressed ubiquitously on the cell surface and in the extracellular matrix. Further, HS in vivo is covalently conjugated to specific core proteins as heparan sulfate proteoglycans (HSPGs). Collectively, these data suggest that the physiological counterpart of anti-inflammatory pharmaceutical heparin is HSPG.
The syndecan family of type I transmembrane HSPGs is the major source of cell surface HS (16, 17). All adherent cells express one or more syndecans on their cell surface. Although all syndecans contain the ligand-binding HS chains, they show distinct temporal and spatial expression patterns and, thus, are likely to function specifically in vivo (16, 18). For example, syndecan-1 is predominantly expressed by epithelial cells and plasma cells in adult tissues, and expressed to a lesser degree by other cell types (e.g. endothelial cells, fibroblasts) (18). All members of the syndecan family are also expressed as soluble HSPGs, because they can be proteolytically cleaved by metalloproteinases and released/secreted into the extracellular environment by ectodomain shedding (16, 17, 19). Syndecan-1 shedding is induced by several inflammatory mediators in vitro and under certain inflammatory conditions in vivo. Examples of syndecan-1 shedding agonists include EGF family growth factors (20), chemokines (21, 22), stress-related agonists (20), heparanase (23), and bacterial virulence factors (24–26). In mouse models of inflammatory diseases, elevated levels of syndecan-1 ectodomains are found in the lung and skin of mice infected with Pseudomonas aeruginosa (27, 28), and in the airway of mice challenged with bleomycin (29) or allergens (30). Further, in humans, elevated levels of syndecan-1 ectodomains are detected in skin wound fluids, serum of patients with acute graft-versus-host disease, and in plasma of myeloma patients, among other fluids from injured or inflamed tissues (31–35).
The physiological function of syndecan-1 shedding has yet to be clearly defined, but accumulating evidence suggests that syndecan-1 shedding modulates the extent and outcome of inflammatory processes. Soluble syndecan-1 ectodomains bind to and modulate various inflammatory factors through their HS moiety. For example, syndecan-1 ectodomains inhibit cell proliferation in response to FGF-2 and HB-EGF by interfering with growth factor binding to cell surface HSPG coreceptors (33). Syndecan-1 ectodomains also bind to the CC chemokines, MARC (CCL7, MCP-3), eotaxin (CCL11), and TARC (CCL17), in an HS-dependent manner, and inhibit the capacity of these CC chemokines to induce Th2 cell migration both in vitro and in vivo (30). Consistent with these data, in a mouse model of allergic lung inflammation, allergen-instilled syndecan-1-null (Sdc1–/–) mice show increased Th2 cell accumulation in the lung compared with wild-type (Wt) mice, and the allergic inflammatory parameters are suppressed by airway administration of purified syndecan-1 ectodomains or HS (30). Further, because soluble HS and heparin bind to and regulate many cytokines (16), syndecan-1 ectodomains are likely to function similarly through their HS chains. Collectively, these data suggest that syndecan-1 shedding is an important post-translational mechanism that regulates the extent and outcome of tissue injury and inflammation by modulating key inflammatory factors.
In this study, we investigated the role of syndecan-1 in modulating the host inflammatory response in Gram-positive toxic shock induced by SEB. SEB is one of at least 13 enterotoxins produced by Staphylococcus aureus. Along with SEC and toxic shock syndrome toxin-1 (TSST-1), SEB is a major cause of non-menstrual staphylococcal toxic shock syndrome (36). Our data show that syndecan-1 shedding is induced in vivo during SEB-induced toxic shock, and syndecan-1 ectodomain attenuates SEB shock by suppressing the amplification of the systemic cytokine storm and T cell-mediated inflammatory tissue injury in an HS-dependent manner. These data suggest that syndecan-1 shedding may be an important host defense mechanism against Gram-positive toxic shock.
EXPERIMENTAL PROCEDURES
Materials—SEB was purchased from Toxin Technology (Sarasota, FL). d-Galactosamine (d-gal), porcine gut mucosal chondroitin sulfate B (CS), and red blood cell lysis buffer were from Sigma-Aldrich. Bovine kidney HS was purchased from MP Biomedicals (Irvine, CA). Recombinant mouse syndecan-1 ectodomain devoid of HS was expressed as a GST fusion protein in Escherichia coli and purified by glutathione affinity chromatography as described previously (28). Purified recombinant syndecan-1 ectodomain was incubated with the endotoxin removal resin (Associates of Cape Cod, East Falmouth, MA) to remove residual LPS, and the absence of LPS was confirmed by the Limulus amebocyte lysate assay (Cambrex, East Rutherford, NJ). Rat anti-mouse CD3 (clone 17A2) and rat anti-mouse CD19 (clone 6D5) monoclonal antibodies were from Biolegend (San Diego, CA), 281-2 rat anti-mouse syndecan-1 monoclonal antibodies were from Pharmingen (San Diego, CA), Ky8.2 rat anti-mouse syndecan-4 monoclonal antibodies were from Dr. Paul Kincade (Oklahoma Medical Research Foundation, Oklahoma City, OK), and Alexa 594 donkey anti-rat antibodies were from Invitrogen Molecular Probes (Carlsbad, CA). The cationic nylon membrane, Immobilon Ny+, was from Millipore (Danvers, MA). ELISA kits for mouse IL-2, IFNγ, TNFα, and IL-6 were obtained from R&D Systems (Minneapolis, MN). Cell strainers (70 μm) for isolation of splenocytes were from Falcon (Franklin Lakes, NJ), and serum collection syringes were from Sarstedt (Newton, NC). RNeasy midi kit was purchased from Qiagen (Valencia, CA) and the Superscript one-step RT-PCR kit was from Invitrogen. Oligonucleotide primers for RT-PCR were obtained from Integrated DNA Technologies (Coralville, IA).
Mouse Model of Gram-positive Toxic Shock—Sdc1–/– mice are healthy with normal growth, reproduction, tissue morphology, CBC counts, and serum chemistry parameters under normal laboratory housing conditions (28, 37, 38). Female Sdc1–/– mice backcrossed eight times onto the BALB/c background and littermate female Wt BALB/c mice were used at an age of 7–9 weeks. Mice were maintained in microisolator cages under specific pathogen-free conditions in a 12-h light/dark cycle and fed a basal rodent chow ad libitum. All animal experiments were approved by the local Institutional Animal Care and Use Committee and complied with federal guidelines for research with experimental animals.
Mice were injected intraperitoneally with 20 mg of d-gal and injected 2 h later with various doses of SEB as indicated. In some experiments, mice were injected intraperitoneally with anti-CD3 antibodies (50 μg/mouse) 24 h prior to SEB injection to induce T lymphocytopenia or injected intraperitoneally with HS, syndecan-1 core protein, or CS 4 h prior to SEB injection. Mice were monitored for signs of distress, and blood and tissue samples were collected for analyses at the indicated times post-SEB injection.
Serum Chemistry—Serum was prepared from blood collected by cardiac puncture into serum collection syringes and serum levels of organ injury and dysfunction markers (ALT, AST, BUN, LDH) were measured using an automated Cobas Integra 400 Plus serum chemistry analyzer (Center for Comparative Medicine, Baylor College of Medicine).
RT-PCR and Splenocyte Assays—Total RNA (200 ng) isolated from lung and liver homogenates with the RNeasy midi kit at 0-, 3-, and 7-h post-SEB was reverse-transcribed into cDNA and amplified using the Superscript one-step RT-PCR kit. The primers used were: 5′-ATG AGA CGC GCG GCG CTC TG-3′ (sense) and 5′-CTG ATT GGC AGT TCC ATC CT-3′ (anti-sense) for syndecan-1, and 5′-GTG GGC CGC TCT AGG CAC CAA-3′ (sense) and 5′-CTC TTT GAT GTC ACG CAC GAT TTC-3′ (antisense) for β-actin. Samples were separated on a 2% agarose gel, visualized by ethidium bromide staining, and photographed.
Splenocytes from Sdc1–/– and Wt mice were isolated by straining spleens through 70-μm screens and lysing erythrocytes with a red blood cell lysis buffer. Splenocytes were resuspended in culture medium (RPMI with 10% fetal calf serum), and 2 × 106 cells were incubated with 1 μg/ml SEB for 24 h at 37 °C. Levels of IL-2, IFNγ, TNFα, and IL-6, in the conditioned medium were determined by ELISA.
Histological Analyses—Wt and Sdc1–/– livers were isolated at various times post-SEB, fixed in 4% paraformaldehyde/phosphate-buffered saline for 2 days at 4 °C, embedded in paraffin, and sectioned. Tissue sections (5 μm) were immunostained with rat anti-mouse CD3 or 281-2 rat anti-mouse syndecan-1 ectodomain and Alexa 594 donkey anti-rat IgG antibodies.
Measurement of Syndecan Ectodomains—Serum levels of syndecan-1 and -4 ectodomains were assessed by a dot immunoblot assay as described previously (24, 25). Briefly, 10–70 μl of serum collected at 0-, 3-, 7-, and 11-h post-SEB were dotblotted onto Immobilon Ny+, and the concentration of shed ectodomains was quantified using 281-2 anti-syndecan-1 or Ky8.2 anti-syndecan-4 ectodomain antibodies.
Statistical Analyses—All data are expressed as mean ± S.E. Differences between experimental groups and respective controls were examined by the Student's t test, and differences in survival values were compared by the Fisher's exact test. p values of <0.05 were considered statistically significant.
RESULTS
Syndecan-1-null Mice Show Enhanced Susceptibility to Lethal SEB Shock—To determine the physiological significance of syndecan-1 in SEB shock, Wt and Sdc1–/– mice on the BALB/c background were injected intraperitoneally with 20 mg/mouse d-gal and 5 μg/mouse SEB 2 h later, and their survival was tracked over the next 5 days. Mice are naturally more resistant to bacterial superantigens than humans because the affinity of superantigens for mouse MHC class II antigens is lower (39). d-gal increases the sensitivity of mice to instigators of Gram-positive toxic shock (40–45). At 5 μg of SEB, all Sdc1–/– mice died by 1-day post-SEB, whereas 100% of Wt mice survived the duration of the experiment (Fig. 1). Essentially the same results were obtained when Sdc1–/– and Wt mice were challenged with 10 μg/mouse SEB, and none died in both Wt and Sdc1–/– groups injected with d-gal only (data not shown). The first signs of illness were seen at about 8–12-h post-SEB challenge, and they included piloerection, decreased mobility, and hunched stature. Thereafter, death was rapid, usually within 4 h of the first signs of distress. These results indicate that the lethal host response to SEB is markedly exacerbated in the absence of syndecan-1, suggesting that syndecan-1 is an endogenous molecule that protects the host from Gram-positive toxic shock.
FIGURE 1.
Sdc1–/– mice are more susceptible to lethal SEB shock. Wt (closed circles) and Sdc1–/– (open circles) mice were injected intraperitoneally with d-gal and 5 μg of SEB, and survival was tracked for 5 days (n = 20 in each group; *, p < 0.05 between Wt and Sdc1–/– mice at ≥1-day post-SEB).
Inflammatory Tissue Injury in SEB Shock Is Exacerbated in Sdc1–/–-null Mice—To determine the physiological basis of enhanced lethality in SEB-injected Sdc1–/– mice, we measured serum levels of various tissue injury and dysfunction markers: alanine aminotransferase (ALT, for liver damage); aspartate aminotransferase (AST, liver and heart); blood urea nitrogen (BUN) and creatinine (kidney); total protein and albumin (vascular leakage, liver damage); creatine kinase (CK, muscle); and lactate dehydrogenase (LDH, cell death). Unless otherwise noted, we injected mice with 20 mg of d-gal/mouse and 5 μg of SEB/mouse 2 h later (d-gal/SEB) (a lethal dose for Sdc1–/– mice but a non-lethal dose for Wt mice) in this experiment and subsequent in vivo studies to maximize differences between the two groups.
Wt and Sdc1–/– mice were injected with d-gal or d-gal/SEB, and blood was collected at 0-, 7-, and 11-h post-injection. Injection with d-gal alone did not affect any of the tissue injury/dysfunction parameters in either Sdc1–/– or Wt mice, and d-gal/SEB did not affect serum BUN, creatinine, and CK in both backgrounds (data not shown). However, relative to Wt mice, serum levels of ALT, AST, and LDH were significantly elevated at 7- and 11-h post-SEB, and the lung wet/dry ratio was significantly elevated at 11-h post-SEB in Sdc1–/– mice (Fig. 2A). Further, serum albumin and total serum protein were significantly decreased at 11-h post-SEB in Sdc1–/– mice relative to Wt mice. Total serum protein concentration was 4.12 ± 0.09 g/dl and 3.07 ± 0.25 g/dl, and serum albumin was 2.98 ± 0.04 g/dl and 2.27 ± 0.12 g/dl in Wt and Sdc1–/– mice, respectively (n = 5 for both groups). Because the half-life of albumin in serum is longer than 24 h, these observations suggested that the reduction in serum albumin and total protein was caused by vascular leakage and not by liver dysfunction. These data suggest that syndecan-1 protects the host from SEB shock by attenuating inflammatory liver injury and dysfunction and vascular leakage in multiple organs.
FIGURE 2.
Sdc1–/– mice show enhanced tissue injury and dysfunction in SEB shock. Wt and Sdc1–/– mice were injected with d-gal/SEB and serum levels of ALT, AST, and LDH, and the lung wet/dry ratio were measured at 0-, 7-, and 11-h post-SEB. Results shown are mean ± S.E. (n = 5; *, p < 0.05 relative to Wt mice at the same time point).
Syndecan-1 Deficiency Amplifies the Systemic Cytokine Storm in SEB Shock—To begin elucidating the molecular basis of how syndecan-1 attenuates SEB shock, we first tested the possibility that syndecan-1 binds to and inhibits SEB, and found that syndecan-1 or HS does not bind to SEB by ligand dot blotting, nitrocellulose capture assay (28), or heparin affinity chromatography (data not shown). We next examined the systemic cytokine response of Sdc1–/– and Wt mice at various times post-SEB injection. The massive systemic release of pro-inflammatory cytokines is causally linked to the pathogenesis of toxic shock syndromes. Several cytokines are essential, because neutralizing antibodies against IFNγ (46, 47) and TNFα (43, 44, 47, 48) are protective in SEB shock. Thus, we explored the possibility that syndecan-1 deficiency led to an exaggeration of the systemic cytokine storm.
At 0-, 3-, 7-, and 11-h post-SEB injection, serum levels of IL-2, IFNγ, TNFα, and IL-6 were measured by ELISA (Fig. 3). Serum IL-2 levels were markedly elevated at 3- and 7-h post-SEB, but did not differ between Wt and Sdc1–/– mice. IFNγ levels also increased progressively, but were similar between the two backgrounds at all times examined. In contrast, both serum TNFα and IL-6 were sharply increased at 3-h post-SEB, and they were significantly higher in Sdc1–/– mice relative to Wt mice (TNFα: 4-fold, IL-6: 2-fold) (Fig. 3). These results suggest that Sdc1–/– mice are more susceptible to SEB shock because of an amplified cytokine storm.
FIGURE 3.
Sdc1–/– mice show amplified systemic production of TNFα and IL-6 in SEB shock. Wt and Sdc1–/– mice were injected with d-gal/SEB and serum levels of IL-2, IFNγ, TNFα, and IL-6 were determined by ELISA at the indicated times. Data shown are mean ± S.E. (n = 5; *, p < 0.05 relative to Wt mice at the same time point).
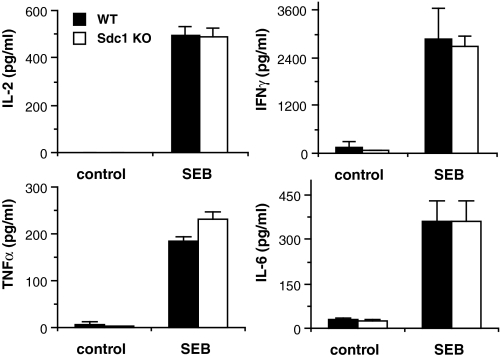
We next assessed if syndecan-1 modulates the cellular cytokine response to SEB by measuring the production of inflammatory cytokines by isolated Sdc1–/– and Wt splenocytes. Wt or Sdc1–/– splenocytes were incubated with culture medium (control) or medium with 1 μg/ml SEB, and the concentration of IL-2, IFNγ, TNFα, and IL-6 in the conditioned medium was determined. Surprisingly, both Sdc1–/– and Wt splenocytes produced similar levels of cytokines in response to SEB (Fig. 4). Consistent with the finding that syndecan-1 does not bind to SEB, these data indicate that syndecan-1 does not directly alter the cytokine-inducing activities of SEB and the ability of T cells and MHC class II-positive cells to respond to SEB. More important, these data indicate that syndecan-1 protects the host from SEB shock by interfering with mechanisms downstream of the initial induction of cytokines by SEB.
FIGURE 4.
Syndecan-1 does not affect the capacity of SEB to stimulate cytokine production. Splenocytes isolated from Wt or Sdc1–/– mice were incubated with culture medium (control) or medium with 1 μg/ml SEB for 24 h, and the concentration of IL-2, IFNγ, TNFα, and IL-6 in the conditioned medium was determined by ELISA (mean ± S.E., n = 4).
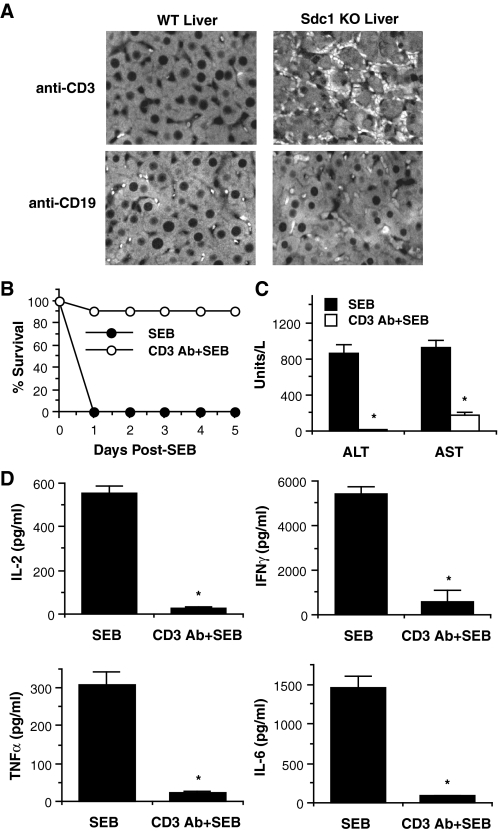
T Cells Mediate the Enhanced Susceptibility of Sdc1–/– Mice to SEB Shock—Because T cells play a central role in SEB shock, we next examined if syndecan-1 deficiency led to dysregulated T cell activities. We initially examined the effects of syndecan-1 deletion on SEB-induced T cell inflammation. Wt and Sdc1–/– mice were injected with d-gal/SEB, and their livers were harvested at 10-h post-SEB. Liver sections were immunostained with rat anti-mouse CD3 or rat anti-mouse CD19 monoclonal antibodies to assess the tissue accumulation of T cells and B cells, respectively (Fig. 5A). Anti-CD19 immunostaining showed minimal accumulation of B cells in SEB-injected Sdc1–/– and Wt livers. Similarly, anti-CD3 staining showed minimal T cell accumulation in Wt livers. The most striking difference was seen in Sdc1–/– livers, which showed markedly increased numbers of CD3-positive T cells in the liver interstitium and microcapillaries compared with Wt livers (Fig. 5A). These data suggest that enhanced T cell accumulation is the cellular cause of amplified liver injury seen in SEB-injected Sdc1–/– mice.
FIGURE 5.
T cells mediate the enhanced susceptibility of Sdc1–/– mice to SEB shock. A, liver sections (5 μm) of Wt and Sdc1–/– mice at 10-h post-SEB were immunostained with anti-CD3 (T cells) or anti-CD19 (B cells) and Alexa 594 antibodies (original magnification, ×200). B, survival of Sdc1–/– mice pretreated with or without 50 μg/mouse anti-CD3 and injected with d-gal/SEB was tracked for 5 d (n = 10). C, serum levels of ALT and AST in Sdc1–/– mice pretreated with or without anti-CD3 and injected with d-gal/SEB were measured by serum chemistry at 7-h post-SEB (n = 6; *, p < 0.05 relative to untreated mice). D, Sdc1–/– mice were pretreated with or without anti-CD3 and injected with d-gal/SEB, and their serum IL-2, IFNγ, TNFα, and IL-6 levels were measured by ELISA at 3-h post-SEB (n = 5; *, p < 0.05 relative to untreated mice).
To further define the functional role of T cells in the enhanced susceptibility of Sdc1–/– mice, we made Sdc1–/– mice T lymphocytopenic and assessed their response to SEB. Acute T lymphocytopenia was achieved by anti-CD3 immunodepletion (49). Untreated or anti-CD3 pretreated Sdc1–/– mice were injected with d-gal/SEB, and their survival was tracked for 5 days. Consistent with previous results, all of the untreated Sdc1–/– mice died by 1-day post-SEB, whereas the T lymphocytopenic Sdc1–/– mice were significantly protected from lethal SEB shock (Fig. 5B). Induced T lymphocytopenia also significantly attenuated liver injury (Fig. 5C) and systemic cytokine production (Fig. 5D) in SEB-injected Sdc1–/– mice. These results indicate that dysregulated T cell activities mediate the amplification of the systemic cytokine storm and exacerbation of inflammatory tissue injury in Sdc1–/– mice undergoing SEB shock.
Syndecan-1 Shedding Is Activated during SEB Shock—Based on these data, we propose that syndecan-1 protects the host from SEB shock by suppressing the T cell-mediated amplification of the cytokine storm and inflammatory tissue damage. To begin elucidating the molecular basis of how syndecan-1 accomplishes these functions, we next examined the expression of syndecan-1 before and after SEB injection.
Wt mice were injected with d-gal or d-gal/SEB, and their livers were harvested at 7-h post-injection. Liver sections were immunostained with the 281-2 rat monoclonal anti-mouse syndecan-1 ectodomain antibody. d-gal-injected Wt liver showed intense cell surface staining of syndecan-1 at hepatocyte-hepatocyte junctions and on the sinusoidal side of hepatocytes, indicating that hepatocytes and possibly microvascular endothelial cells express syndecan-1 (Fig. 6A). These staining patterns were similar to those of unchallenged Wt liver (not shown), indicating that d-gal does not affect the expression pattern of syndecan-1. However, when Wt mice were injected with d-gal/SEB, the intense cell surface staining of syndecan-1 in the liver was abolished (Fig. 6A). Similar results were seen in Wt lungs where staining of cell surface syndecan-1 on cuboidal type II epithelial cells was dramatically reduced at 7-h post-SEB injection (not shown), suggesting that SEB induces syndecan-1 shedding in multiple organs. Further, because steady-state syndecan-1 mRNA levels were not decreased in the liver at 3- and 7-h post-SEB (Fig. 6B), the reduced surface expression suggested that syndecan-1 ectodomains are shed during SEB shock.
FIGURE 6.
Syndecan-1 shedding is induced in SEB shock. A, livers harvested from Wt mice injected with d-gal or d-gal/SEB at 7-h post-injection were embedded in paraffin and tissue sections (5 μm) were immunostained for syndecan-1 (original magnification, ×200). B, syndecan-1 and β-actin mRNA in Wt livers isolated at the indicated times post-SEB were measured by RT-PCR. C, serum levels of syndecan-1 and -4 ectodomains were measured by dot immunoblotting (n = 4; *, p < 0.05).
We next examined whether syndecan-1 ectodomains are indeed shed during SEB shock by measuring serum levels of syndecan-1 ectodomains. Wt mice were injected with d-gal/SEB, and serum syndecan-1 and -4 ectodomains were quantified at 0-, 3-, 7-, and 11-h post-SEB. Serum syndecan-1 ectodomains increased significantly and progressively between 3- and 11-h post-SEB, reaching a value 30-fold higher than baseline at 11-h post-SEB (Fig. 6C). Serum syndecan-4 ectodomains were not elevated at all times, suggesting that syndecan-1 shedding is specifically activated during SEB shock. Further, serum syndecan-1 ectodomains were not detected when probed with antibodies directed against the syndecan-1 cytoplasmic domain (24), verifying that the ectodomains were shed and not released intact from damaged cells (not shown). These results indicate that syndecan-1 ectodomains are specifically shed from the liver and released into the circulation. Moreover, these data suggest that syndecan-1 shedding modulates the host response in SEB shock.
Syndecan-1 Ectodomain Attenuates SEB Shock through Its HS Moiety—We reasoned that if the activation of syndecan-1 shedding is indeed important in the attenuation of SEB shock, then the administration of syndecan-1 ectodomain analogues should rescue Sdc1–/– mice from the enhanced susceptibility to SEB shock. We first tested this by determining the effects of the three moieties of syndecan-1 ectodomain (HS, syndecan-1 core protein, and CS) on lethal SEB shock.
Sdc1–/– mice were injected intraperitoneally with saline or with 10 or 50 μg/mouse of HS, syndecan-1 core protein, or CS 4 h prior to SEB injection, injected with d-gal/SEB, and their survival was tracked for 5 days (Fig. 7). All mice in the group injected with saline then d-gal/SEB died by 1-day post-SEB. Similarly, all mice in the groups pretreated with 50 μg of syndecan-1 core protein or CS died by 2-days post-SEB. However, only 20% of Sdc1–/– mice that were pretreated with 50 μg of HS and injected with d-gal/SEB died during the course of the experiment (Fig. 7). At 10 μg/mouse, HS also showed a trend toward improving the odds of survival (3/10 survival), but again, all mice in groups pretreated with 10 μg/mouse syndecan-1 core protein or CS died by 1-day post-SEB (not shown). No abnormal effects, including liver injury and lethality, were seen in mice injected with 50 μg of HS, syndecan-1 core protein, or CS only. Johnson et al. (50) found that injection of HS induces SIRS-like features in mice, but this apparent discordant observation was most likely caused by the fact that they used 100-fold more HS (5 mg/mouse) in their studies. Altogether, these data indicate that syndecan-1 ectodomains inhibit lethal SEB shock in an HS-dependent manner.
FIGURE 7.
Syndecan-1 ectodomain inhibits lethal SEB shock in an HS-dependent manner. Sdc1–/– mice were pretreated intraperitoneally with or without 50 μg of HS, syndecan-1 core protein, or CS, injected with d-gal/SEB, and their survival was tracked for 5 days (n = 20 for HS group, n = 10 for other groups; *, p < 0.05 between HS pretreated group and other groups at ≥1-day post-SEB).
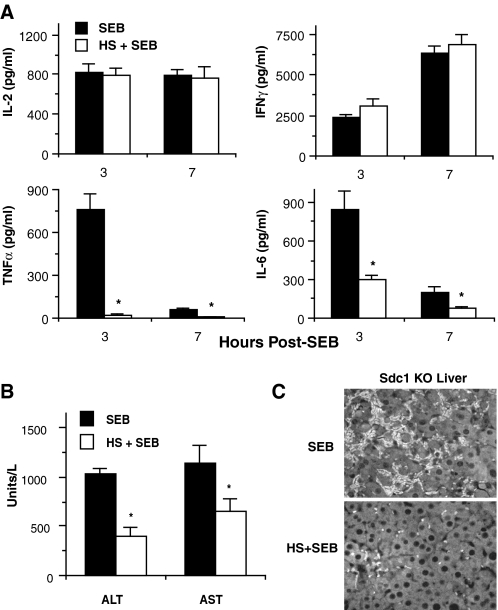
We next examined the effects of HS on SEB-induced cytokine production, liver injury, and T cell accumulation in the liver. Sdc1–/– mice were pretreated with or without HS and injected with d-gal/SEB. HS treatment did not affect serum IL-2 or IFNγ levels, but it abrogated the TNFα response and significantly reduced IL-6 levels by ∼4-fold (Fig. 8A). HS also significantly reduced both serum ALT and AST levels (Fig. 8B) and markedly dampened the accumulation of T cells in the liver (Fig. 8C). Because these parameters were increased in SEB-injected Sdc1–/– mice relative to Wt mice (Figs. 2, 3, and 5A), these data indicate that the HS moiety of syndecan-1 ectodomain attenuates SEB shock by specifically inhibiting the amplified production of TNFα and IL-6, and T cell-mediated inflammatory tissue injury.
FIGURE 8.
HS attenuates the amplification of TNFα and IL-6 production and T cell-mediated liver injury in SEB shock. Sdc1–/– mice were pretreated with or without HS and serum levels of cytokines were measured by ELISA at the indicated times (A) and serum ALT and AST were measured by serum chemistry at 7-h post-SEB (B)(n = 5, *, p < 0.05 relative to mice that received d-gal/SEB only). C, liver sections (5 μm) of Sdc1–/– mice treated as indicated and isolated at 10-h post-SEB were immunostained with anti-CD3 (original magnification, ×200).
DISCUSSION
Gram-positive toxic shock is a serious systemic disease with progressive stages. However, not all patients with invasive staphylococcal or streptococcal diseases develop toxic shock, suggesting that endogenous mechanisms that attenuate disease progression might exist. Indeed, several endogenous factors that protect the host from toxic shock have been identified, such as IL-10 (46), Fas (44), and CD44 (42). Our current study adds syndecan-1 to the short list of endogenous inhibitors of Gram-positive toxic shock. Our data showed that syndecan-1 shedding is induced in SEB shock and that syndecan-1 ectodomains attenuate SEB shock by specifically inhibiting the overproduction of TNFα and IL-6, accumulation of T cells in tissues, and subsequent T cell-mediated inflammatory tissue injury in an HS-dependent manner. Consistent with these findings, mice lacking syndecan-1 were more prone to pathological features of SEB shock compared with Wt mice. SEB-injected Sdc1–/– mice showed significantly elevated levels of systemic TNFα and IL-6, markedly increased accumulation of T cells, and exaggerated liver injury and vascular permeability relative to SEB-injected Wt mice. Further, Sdc1–/– mice were significantly more susceptible to lethal shock at low SEB concentrations where the majority of Wt mice were protected. Because other HSPGs, including other syndecans, are intact in Sdc1–/– mice, our data highlight that syndecan-1 functions specifically in attenuating SEB shock because its ectodomain is specifically shed during SEB shock. Altogether, these findings indicate that syndecan-1 is a critical in vivo suppressor of Gram-positive toxic shock.
The underlying mechanism of how syndecan-1 shedding is activated in Gram-positive toxic shock remains to be defined. SEB does not activate syndecan-1 shedding in cultured mouse mammary gland (NMuMG) or lung (LA4) epithelial cells, or isolated splenocytes (not shown), suggesting that factors induced by SEB signaling stimulate syndecan-1 shedding. Syndecan-1 shedding is a highly regulated process that is induced by several inflammatory factors in vitro and under certain inflammatory conditions in vivo (16, 17, 19). Of the cytokines induced during SEB shock, TNFα and IFNγ have been shown to synergistically stimulate syndecan-1 shedding by cultured human enterocytes (51). Our study suggested that the primary tissue source of circulating syndecan-1 ectodomains is the liver. Thus, in light of the fact that TNFα is the effector cytokine that causes liver damage and the liver is the principle target organ in the mouse model of SEB shock (42, 43, 48), it is plausible that SEB-induced TNFα and IFNγ synergistically trigger syndecan-1 shedding in the liver in mice undergoing Gram-positive toxic shock.
How TNFα and IFNγ synergize to induce syndecan-1 shedding is not known, but Bode et al. (52) recently showed that IFNγ potentiates the activity of TNFα by up-regulating the expression of TNFR1. In addition, TNFα induces the expression of MMP-7 (53), the metalloproteinase that sheds syndecan-1 ectodomains both in vitro (54) and in vivo from the surface of activated airway epithelium (29). Collectively, these findings suggest a putative shedding mechanism in Gram-positive toxic shock where IFNγ potentiates TNFα by up-regulating the expression of TNFR1, which in turn induces MMP-7 to stimulate MMP-7-mediated syndecan-1 shedding at the cell surface.
Our study showed that syndecan-1 shedding protects the host from SEB shock by dampening the overproduction of TNFα and IL-6, and T cell-mediated inflammatory tissue injury in an HS-dependent manner. Syndecan-1 ectodomain does not bind to SEB, and its absence or presence does not directly affect the SEB-induced production of IL-2, IFNγ, TNFα, and IL-6 by isolated splenocytes, suggesting that syndecan-1 ectodomain HS inhibits mechanisms downstream of SEB signaling that amplify the production of TNFα and IL-6, and augment T cell accumulation in tissues. Further, because systemic levels of the T cell cytokines, IL-2 and IFNγ, were similar between Wt and Sdc1–/– mice, the inhibitory effects of syndecan-1 ectodomain HS are also downstream of T cell activation by SEB. Precisely how syndecan-1 ectodomain HS suppresses certain components of the cytokine storm and T cell tissue inflammation in SEB shock remains to be determined, but several criteria suggest that syndecan-1 ectodomains accomplish these protective functions by interfering with T cell-derived IFNγ in an HS-dependent manner.
First, our studies indicated that T cells are required for the production of TNFα and IL-6 during SEB shock in vivo. Despite the fact that SEB can activate MHC class II-positive APCs to produce TNFα and IL-6, among other cytokines, our studies showed that T cell immunodepletion significantly reduces systemic levels of not only IL-2 and IFNγ, but also TNFα and IL-6. Second, IFNγ can induce the expression of TNFα (55, 56) and IL-6 (57) and transgenic mice overexpressing IFNγ develop chronic hepatitis because of elevated TNFα (58), suggesting that T cell-derived IFNγ amplifies the production of TNFα and IL-6 in SEB shock. Further, IFNγ is a potent physiological inducer of the CXC chemokines, MIG (monokine induced by IFNγ, CXCL9), IP-10 (IFNγ-induced protein, CXCL10), and I-TAC (interferon-inducible T cell alpha chemoattractant, CXCL11), which are the primary chemoattractants of activated T cells (59–61). Third, HS and heparin bind to and inhibit IFNγ (8, 9, 62). Altogether, these observations suggest that the HS moiety of syndecan-1 ectodomain attenuates SEB shock by inhibiting the capacity of IFNγ to amplify TNFα and IL-6 production, and facilitate the recruitment of SEB-activated T cells by inducing the expression of T cell chemotactic CXC chemokines.
SEB is one of several staphylococcal pyrogenic superantigens implicated in staphylococcal toxic shock syndrome. SEB, SEC, and TSST-1 cause the majority of non-menstrual toxic shock syndrome cases, whereas TSST-1 is the primary cause of menstrual toxic shock syndrome (63). Staphylococcal superantigens are also associated with other immune and inflammatory disorders, such as food poisoning, arthritis, and sepsis (1). Although encoded by distinct genes and, despite a variable homology of 22–80% in their primary sequence, staphylococcal superantigens share a similar overall three-dimensional structure and instigate similar dysregulated inflammatory responses (1, 3, 4). Future studies directed at determining the effects of syndecan-1 on toxic shock induced by other staphylococcal superantigens and other superantigen-induced diseases should reveal if syndecan-1 is a broadly used endogenous antidote against inflammatory disorders instigated by staphylococcal pyrogenic superantigens.
Acknowledgments
We thank Su Gu, Sheila Duncan, and Atsuko Hayashida for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL69050 and HL81474. This work was also supported by a Research Award from the Mizutani Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SIRS, systemic inflammatory response syndrome; ALT, alanine aminotransferase; APC, antigen-presenting cell; AST, aspartate aminotransferase; CS, chondroitin sulfate; d-gal, d-galactosamine; HS, heparan sulfate; HSPG, heparan sulfate proteoglycan; MHC, major histocompatibility complex; SEB, staphylococcal enterotoxin B; TCR, T cell receptor; TSST-1, toxic shock syndrome toxin-1; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; ELISA, enzyme-linked immunosorbent assay; Wt, wild type.
References
- 1.Kotb, M. (1995) Clin. Microbiol. Rev. 8 411–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrack, P., and Kappler, J. (1990) Science 248 705–711 [DOI] [PubMed] [Google Scholar]
- 3.McCormick, J. K., Yarwood, J. M., and Schlievert, P. M. (2001) Annu. Rev. Microbiol. 55 77–104 [DOI] [PubMed] [Google Scholar]
- 4.Dinges, M. M., Orwin, P. M., and Schlievert, P. M. (2000) Clin. Microbiol. Rev. 13 16–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, M. R., and Tary-Lehmann, M. (2000) Clin. Immunol. 98 85–94 [DOI] [PubMed] [Google Scholar]
- 6.Najjam, S., Mulloy, B., Theze, J., Gordon, M. Y., Gibbs, R. V., and Rider, C. C. (1998) Glycobiology 8 509–516 [DOI] [PubMed] [Google Scholar]
- 7.Wrenshall, L. E., Platt, J. L., Stevens, E. T., Wight, T. N., and Miller, J. D. (2003) J. Immunol. 170 5470–5474 [DOI] [PubMed] [Google Scholar]
- 8.Lortat-Jacob, H., Turnbull, J. E., and Grimaud, J. A. (1995) Biochem. J. 310 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarrazin, S., Bonnaffe, D., Lubineau, A., and Lortat-Jacob, H. (2005) J. Biol. Chem. 280 37558–37564 [DOI] [PubMed] [Google Scholar]
- 10.Lantz, M., Thysell, H., Nilsson, E., and Olsson, I. (1991) J. Clin. Investig. 88 2026–2031 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Mummery, R. S., and Rider, C. C. (2000) J. Immunol. 165 5671–5679 [DOI] [PubMed] [Google Scholar]
- 12.Tyrrell, D. J., Horne, A. P., Holme, K. R., Preuss, J. M., and Page, C. P. (1999) Adv. Pharmacol. 46 151–208 [DOI] [PubMed] [Google Scholar]
- 13.Day, R., and Forbes, A. (1999) Lancet 354 62–65 [DOI] [PubMed] [Google Scholar]
- 14.Davidson, B. L., Geerts, W. H., and Lensing, A. W. (2002) N. Engl. J. Med. 347 1036–1037 [DOI] [PubMed] [Google Scholar]
- 15.Wang, L., Brown, J. R., Varki, A., and Esko, J. D. (2002) J. Clin. Investig. 110 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernfield, M., Götte, M., Park, P. W., Reizes, O., Fitzgerald, M. L., Lincecum, J., and Zako, M. (1999) Annu. Rev. Biochem. 68 729–777 [DOI] [PubMed] [Google Scholar]
- 17.Park, P. W., Reizes, O., and Bernfield, M. (2000) J. Biol. Chem. 275 29923–29926 [DOI] [PubMed] [Google Scholar]
- 18.Bernfield, M., Kokenyesi, R., Kato, M., Hinkes, M. T., Spring, J., Gallo, R. L., and Lose, E. J. (1992) Annu. Rev. Cell Biol. 8 365–393 [DOI] [PubMed] [Google Scholar]
- 19.Bartlett, A. H., Hayashida, K., and Park, P. W. (2007) Mol. Cell 24 153–166 [PubMed] [Google Scholar]
- 20.Fitzgerald, M. L., Wang, Z., Park, P. W., Murphy, G., and Bernfield, M. (2000) J. Cell Biol. 148 811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charnaux, N., Brule, S., Chaigneau, T., Saffar, L., Sutton, A., Hamon, M., Prost, C., Lievre, N., Vita, C., and Gattegno, L. (2005) Glycobiology 15 119–130 [DOI] [PubMed] [Google Scholar]
- 22.Brule, S., Charnaux, N., Sutton, A., Ledoux, D., Chaigneau, T., Saffar, L., and Gattegno, L. (2006) Glycobiology 16 488–501 [DOI] [PubMed] [Google Scholar]
- 23.Yang, Y., Macleod, V., Miao, H. Q., Theus, A., Zhan, F., Shaughnessy, J. D., Jr., Sawyer, J., Li, J. P., Zcharia, E., Vlodavsky, I., and Sanderson, R. D. (2007) J. Biol. Chem. 282 13326–13333 [DOI] [PubMed] [Google Scholar]
- 24.Park, P. W., Foster, T. J., Nishi, E., Duncan, S. J., Klagsbrun, M., and Chen, Y. (2004) J. Biol. Chem. 279 251–258 [DOI] [PubMed] [Google Scholar]
- 25.Park, P. W., Pier, G. B., Preston, M. J., Goldberger, O., Fitzgerald, M. L., and Bernfield, M. (2000) J. Biol. Chem. 275 3057–3064 [DOI] [PubMed] [Google Scholar]
- 26.Chung, M. C., Popova, T. G., Millis, B. A., Mukherjee, D. V., Zhou, W., Liotta, L. A., Petricoin, E. F., Chandhoke, V., Bailey, C., and Popov, S. G. (2006) J. Biol. Chem. 281 31408–31418 [DOI] [PubMed] [Google Scholar]
- 27.Haynes, A., 3rd, Ruda, F., Oliver, J., Hamood, A. N., Griswold, J. A., Park, P. W., and Rumbaugh, K. P. (2005) Infect. Immun. 73 7914–7921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park, P. W., Pier, G. B., Hinkes, M. T., and Bernfield, M. (2001) Nature 411 98–102 [DOI] [PubMed] [Google Scholar]
- 29.Li, Q., Park, P. W., Wilson, C. L., and Parks, W. C. (2002) Cell 111 635–646 [DOI] [PubMed] [Google Scholar]
- 30.Xu, J., Park, P. W., Kheradmand, F., and Corry, D. B. (2005) J. Immunol. 174 5758–5765 [DOI] [PubMed] [Google Scholar]
- 31.Joensuu, H., Anttonen, A., Eriksson, M., Makitaro, R., Alfthan, H., Kinnula, V., and Leppa, S. (2002) Cancer Res. 62 5210–5217 [PubMed] [Google Scholar]
- 32.Kainulainen, V., Wang, H., Schick, C., and Bernfield, M. (1998) J. Biol. Chem. 273 11563–11569 [DOI] [PubMed] [Google Scholar]
- 33.Kato, M., Wang, H., Kainulainen, V., Fitzgerald, M. L., Ledbetter, S., Ornitz, D. M., and Bernfield, M. (1998) Nat. Med. 4 691–697 [DOI] [PubMed] [Google Scholar]
- 34.Seidel, C., Ringdén, O., and Remberger, M. (2003) Transplantation 76 423–426 [DOI] [PubMed] [Google Scholar]
- 35.Yang, Y., Yaccoby, S., Liu, W., Langford, J. K., Pumphrey, C. Y., Theus, A., Epstein, J., and Sanderson, R. D. (2002) Blood 100 610–617 [DOI] [PubMed] [Google Scholar]
- 36.Bohach, G. A., and Foster, T. J. (2000) in Gram-positive Pathogens (Fischetti, V. A., Novick, R. P., Ferretti, J. J., Portnoy, D. A., and Rood, J. I., eds) pp. 367–379, American Society for Microbiology, Washington, DC
- 37.Alexander, C. M., Reichsman, F., Hinkes, M. T., Lincecum, J., Becker, K. A., Cumberledge, S., and Bernfield, M. (2000) Nat. Genet. 25 329–332 [DOI] [PubMed] [Google Scholar]
- 38.Stepp, M. A., Gibson, H. E., Gala, P. H., Iglesia, D. D., Pajoohesh-Ganji, A., Pal-Ghosh, S., Brown, M., Aquino, C., Schwartz, A. M., Goldberger, O., Hinkes, M. T., and Bernfield, M. (2002) J. Cell Sci. 115 4517–4531 [DOI] [PubMed] [Google Scholar]
- 39.Li, H., Llera, A., Malchiodi, E. L., and Mariuzza, R. A. (1999) Annu. Rev. Immunol. 17 435–466 [DOI] [PubMed] [Google Scholar]
- 40.Arad, G., Levy, R., Hillman, D., and Kaempfer, R. (2000) Nat. Med. 6 414–421 [DOI] [PubMed] [Google Scholar]
- 41.Bean, A. G. D., Freiberg, R. A., Andrade, S., Menon, S., and Zlotnik, A. (1993) Infect. Immun. 61 4937–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKallip, R. J., Fisher, M., Gunthert, U., Szakal, A. K., Nagarkatti, P. S., and Nagarkatti, M. (2005) Infect. Immun. 73 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miethke, T., Wahl, C., Heeg, K., Echtenacher, B., Krammer, P. H., and Wagner, H. (1992) J. Exp. Med. 175 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mountz, J. D., Baker, T. J., Borcherding, D. R., Bluethmann, H., Zhou, T., and Edwards III, C. K. (1995) J. Immunol. 155 4829–4837 [PubMed] [Google Scholar]
- 45.Saha, B., Harlan, D. M., Lee, K. P., June, C. H., and Abe, R. (1996) J. Exp. Med. 183 2675–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florquin, S., Amraoui, Z., Abramowicz, D., and Goldman, M. (1994) J. Immunol. 153 2618–2623 [PubMed] [Google Scholar]
- 47.Zhang, W. J., Sarawar, S. R., Nguyen, P., Daly, K., Rehg, J. E., Doherty, P. C., Woodland, D. L., and Blackman, M. A. (1996) J. Immunol. 157 5049–5060 [PubMed] [Google Scholar]
- 48.Faulkner, L., Cooper, A., Fantino, C., Altmann, D. M., and Sriskandan, S. (2005) J. Immunol. 175 6870–6877 [DOI] [PubMed] [Google Scholar]
- 49.Tang, Q., Smith, J. A., Szot, G. L., Zhou, P., Alegre, M.-L., Henriksen, K. J., Thompson, C. B., and Bluestone, J. A. (2003) J. Immunol. 170 1510–1516 [DOI] [PubMed] [Google Scholar]
- 50.Johnson, G. B., Brunn, G. J., and Platt, J. L. (2004) J. Immunol. 172 20–24 [DOI] [PubMed] [Google Scholar]
- 51.Henry-Stanley, M. J., Zhang, B., Erlandsen, S. L., Wells, C. L. (2006) Cytokine 34 252–259 [DOI] [PubMed] [Google Scholar]
- 52.Bode, L., Salvestrini, C., Park, P. W., Li, J., Esko, J. D., Yamaguchi, Y., Murch, S., and Freeze, H. H. (2008) J. Clin. Investig. 118 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez-Boado, Y. S., Wilson, C. L., Hooper, L. V., Gordon, J. I., Hultgren, S. J., and Parks, W. C. (2000) J. Cell Biol. 148 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding, K., Lopez-Burks, M., Sanchez-Duran, J. A., Korc, M., and Lander, A. D. (2005) J. Cell Biol. 171 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossman, D., Lamphear, J. G., Mollick, J. A., Betley, M. J., and Rich, R. R. (1992) Infect. Immun. 60 5190–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philip, R., and Epstein, L. B. (1986) Nature 323 86–89 [DOI] [PubMed] [Google Scholar]
- 57.McIntosh, J. K., Jablons, D. M., Mule, J. J., Nordan, R. P., Rudikoff, S., Lotze, M. T., and Rosenberg, S. A. (1989) J. Immunol. 143 162–167 [PubMed] [Google Scholar]
- 58.Simpson, K. J., Lukacs, N. W., Colletti, L., Strieter, R. M., and Kunkel, S. L. (1997) J. Hepatol. 27 1120–1132 [DOI] [PubMed] [Google Scholar]
- 59.Farber, J. M. (1997) J. Leukoc. Biol. 61 246–257 [PubMed] [Google Scholar]
- 60.Rollins, B. J. (1997) Blood 90 909–928 [PubMed] [Google Scholar]
- 61.Simpson, K. J., Henderson, N. C., Bone-Larson, C. L., Lukacs, N. W., Hogaboam, C. M., and Kunkel, S. L. (2003) Clin. Sci. (Lond) 104 47–63 [DOI] [PubMed] [Google Scholar]
- 62.Fritchley, S. J., Kirby, J. A., and Ali, S. (2000) Clin. Exp. Immunol. 120 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bohach, G. A., Fast, D. J., Nelson, R. D., and Schlievert, P. M. (1990) Crit. Rev. Microbiol. 17 251–272 [DOI] [PubMed] [Google Scholar]