Abstract
Human retinol dehydrogenase 10 (RDH10) was implicated in the oxidation of all-trans-retinol for biosynthesis of all-trans-retinoic acid, however, initial assays suggested that RDH10 prefers NADP+ as a cofactor, undermining its role as an oxidative enzyme. Here, we present evidence that RDH10 is, in fact, a strictly NAD+-dependent enzyme with multisubstrate specificity that recognizes cis-retinols as well as all-trans-retinol as substrates. RDH10 has a relatively high apparent Km value for NAD+ (∼100 μm) but the lowest apparent Km value for all-trans-retinol (∼0.035 μm) among all NAD+-dependent retinoid oxidoreductases. Due to its high affinity for all-trans-retinol, RDH10 exhibits a greater rate of retinol oxidation in the presence of cellular retinol-binding protein type I (CRBPI) than human microsomal RoDH4, but like RoDH4, RDH10 does not recognize retinol bound to CRBPI as a substrate. Consistent with its preference for NAD+, RDH10 functions exclusively in the oxidative direction in the cells, increasing the levels of retinaldehyde and retinoic acid. Targeted small interfering RNA-mediated silencing of endogenous RDH10 or RoDH4 expression in human cells results in a significant decrease in retinoic acid production from retinol, identifying both human enzymes as physiologically relevant retinol dehydrogenases. The dual cis/trans substrate specificity suggests a dual physiological role for RDH10: in the biosynthesis of 11-cis-retinaldehyde for vision as well as the biosynthesis of all-trans-retinoic acid for differentiation and development.
Retinoic acid is a small lipophilic molecule derived from vitamin A that regulates gene expression through binding to nuclear transcription factors, retinoic acid receptors (1). The oxidation of retinol to retinaldehyde is the rate-limiting step in retinoic acid biosynthesis (2); however, the identity of the physiologically relevant retinol dehydrogenase(s) has long been controversial. Two types of enzymes have been implicated in the oxidation of retinol: cytosolic alcohol dehydrogenases (ADH)2 of the medium-chain alcohol dehydrogenase superfamily and microsomal dehydrogenases (RoDH) of the short-chain dehydrogenase/reductase (SDR) superfamily (reviewed in Ref. 3). Several criteria have been suggested for evaluation of the candidate retinoid oxidoreductases. First of all, it was proposed that to function in the oxidative direction in vivo, the enzyme has to prefer NAD+ as a cofactor (reviewed in Ref. 4), because unlike NADP+, NAD+ exists mostly in the oxidized form in the majority of cells (5). Second, it was argued that the physiologically relevant retinol dehydrogenase must recognize retinol bound to cellular retinol-binding protein type I (holoCRBPI) as a substrate, because holoCRBPI is the predominant form of retinol in the cells (2). Finally, the enzyme essential for the oxidation of retinol to retinaldehyde was expected to be expressed in the “hot spots” of retinoic acid biosynthesis during various stages of development and be relatively well conserved to support retinoic acid biosynthesis across all vertebrate species (4).
Neither of the currently known retinoid oxidoreductases appears to satisfy all of these requirements (4). For example, microsomal rat retinol dehydrogenase, RoDH1, recognizes holoCRBPI as a substrate, but prefers NADP+ as a cofactor (6). In addition, RoDH1 is not particularly well conserved across species (7). The cytosolic retinoid-active ADHs are NAD+-dependent, but individual ADH isozymes are not present in all “hot spots” of retinoic acid biosynthesis and do not recognize holoCRBPI as a substrate (4, 8). To date, genes for several of the candidate retinol dehydrogenases have been selectively disrupted in mice (9–13); however, neither gene from the ADH or SDR superfamilies was found to be individually required for retinoic acid biosynthesis, suggesting that either retinol dehydrogenases are redundant or the essential enzyme has not yet been identified.
Thus far, the only enzyme proven to be important for retinol oxidation in vivo is the NAD+-dependent 11-cis-retinol dehydrogenase (11-cis-RDH, RDH5), which catalyzes the oxidation of 11-cis-retinol for regeneration of visual pigment 11-cis-retinal in retinal pigment epithelium (14). Mutations in human 11-cis-RDH were linked to fundus albipunctatus, a disease characterized by profound delayed dark adaptation of cone and rod cells (15). One of the features that distinguish 11-cis-RDH from other NAD+-dependent retinol dehydrogenases is the remarkably high degree of 11-cis-RDH conservation across all vertebrate species (7), probably reflecting its physiological importance. The only other retinoid oxidoreductase with a similar degree of conservation is retinol dehydrogenase 10 (RDH10) (16). However, initial assays of recombinant RDH10 activity in COS cells suggested that RDH10 strongly prefers NADP+ over NAD+ as a cofactor (16), contradicting one of the major requirements for an oxidative enzyme. Nevertheless, mutations in murine Rdh10 were recently linked to embryonic lethality in mice treated with N-ethyl-N-nitrosourea (17), suggesting that Rdh10 may be essential for retinoic acid biosynthesis during embryonic development. Here, to clarify the role of RDH10 in retinoid metabolism and to evaluate the relative potential of other candidate SDRs as retinol dehydrogenases, we investigated the cofactor and substrate specificity of RDH10; examined its ability to utilize holoCRBPI as a substrate; and assessed the contribution of RDH10 and similar SDRs to retinoic acid biosynthesis in living human cells.
EXPERIMENTAL PROCEDURES
RDH10 Expression Constructs—The full-length RDH10 cDNA (2751 bp) in pCMV-SPORT vector was obtained from the American Type Culture Collection (Manassas, VA, IMAGE clone number 6153778). To prepare a vector for expression of RDH10 in HEK293 cells, an XhoI restriction site was constructed on the 5′-end of RDH10 cDNA through subcloning into pBluescriptSK(-) plasmid (Stratagene, La Jolla, CA). RDH10 was excised from RDH10-pBluescriptSK(-) plasmid with XhoI and NotI endonucleases and cloned into the matching sites (SalI-NotI) of pCMV-HA vector (Clontech Laboratories, Mountain View, CA) in-frame with the N-terminal HA tag. To express untagged RDH10, a ∼1.5-kb EcoRI-EcoRI fragment containing the full-length RDH10 coding sequence, 5′-untranslated sequence, and partial 3′-untranslated sequence was transferred from the IMAGE clone into the pCMV-Tag4a vector (Stratagene).
For expression in Sf9 cells, the RDH10 coding sequence was PCR-amplified using oligonucleotide primers 5′-GGC TCT AGA ATG AAC ATC GTG GTG GAG TTC TTC G-3′ (sense) and 5′-TGC AAG CTT GAT TCC ATT TTT TGC TTC ATT ATT GTT TGT G-3′ (antisense) to introduce XbaI and HindIII restriction sites, respectively (underlined). This PCR product was subcloned between the XbaI and HindIII sites of pET28a vector (Novagen, Madison, WI) as an intermediate step before transferring the RDH10 cDNA into the SmaI-NotI sites of the pVL1393 vector modified as described previously (18, 19). The final construct of RDH10 in pVL1393-encoded RDH10 fused with the His6 tag on the C terminus. All cDNA constructs were verified by sequencing.
Activity Assays and Kinetic Characterization—Kinetic characterization of RDH10 catalytic properties was carried out using the microsomal preparation of the recombinant enzyme obtained from Sf9 cells. RDH10-His6 was expressed in Sf9 cells using the Baculovirus expression system (Orbigen Inc., San Diego, CA) as described previously for other retinoid-active microsomal SDRs (18–20). Microsomes containing RDH10 were isolated by differential centrifugation, resuspended in 90 mm potassium phosphate, pH 7.4, 40 mm potassium chloride (reaction buffer), supplemented with 1 mm dithiothreitol and 20% glycerol, and stored in small aliquots at -80 °C (20).
Determination of kinetic constants of RDH10 was performed as described previously in detail for RDH12 and RDH11 (18, 19). All-trans-retinol, all-trans-retinaldehyde, and 9-cis-retinaldehyde were obtained from Sigma and purified by high-pressure liquid chromatography (HPLC). 11-cis-Retinaldehyde was a kind gift from Dr. Rosalie Crouch (Medical University of South Carolina, Charleston, SC). 9-cis-Retinol and 11-cis-retinol were synthesized by KBH4 reduction of the corresponding retinaldehydes, followed by HPLC purification. For activity assays, the required amount of retinoid in ethanol was added to the reaction buffer supplemented with equimolar delipidated bovine serum albumin and the mixture was sonicated for 10 min to disperse retinoids. The actual amount of solubilized retinoid was determined based on the corresponding molar absorption coefficients at the appropriate wavelength. The following molar absorption coefficients in aqueous solutions were used: ε328 = 39500 m-1 cm-1 for all-trans-retinol, ε326 = 39733 m-1 cm-1 for 9-cis-retinol, and ε329 = 25800 m-1 cm-1 for 11-cis-retinol (21). The reactions were carried out in siliconized glass tubes for 15 min at 37 °C and stopped by the addition of an equal volume of methanol. Retinoids were extracted twice with 2 volumes of hexane, dried under N2 gas, reconstituted into hexane, and separated by normal phase HPLC on a Spherisorb S3W column (4.6 × 100 mm) (Waters Corp., Milford, MA) in hexane/ethyl acetate (90:10, v/v) mobile phase, at a flow rate of 0.7 ml/min using the Waters Alliance 2695 HPLC system equipped with a Waters 2996 photodiode array.
The apparent Km values for retinols were determined at 1 mm NAD+ and 5–6 concentrations (0.0625–2 μm) of all-trans-retinol, 9-cis-retinol, or 11-cis-retinol. The apparent Km value for all-trans-retinaldehyde was determined at 1 mm NADH and six concentrations of all-trans-retinaldehyde (0.125–8 μm). The apparent Km value for NAD+ was determined at saturating all-trans-retinol (5 μm) and 5–6 concentrations of cofactor (1–625 μm). The background value without cofactor was determined for each concentration of substrate and was subtracted from each data point. The amount of protein varied between 3 and 20 μg, so that the amount of the product did not exceed 10% of the substrate. Reaction rates were determined based on the percent substrate conversion as described previously (18, 19). Kinetic constants were calculated using GraFit (Erithacus Software Ltd., UK) and expressed as the mean ± S.D. The results shown are representative of three to four experiments.
ApoCRBPI and holoCRBPI were prepared as described previously (19). The A350/A280 ratio of the holo-CRBPI was 1.75. Sf9 microsomes containing recombinant human RoDH4 were prepared as described in Gough et al. (20).
Overexpression of RDH10—HEK293 cells (American Type Culture Collection, Manassas, VA) were selected for these experiments because they are well characterized in terms of retinoid metabolism (22, 23). All RDH10 overexpression experiments were conducted in comparison with mock-transfected cells.
HEK293 cells were cultured in minimal essential medium containing 10% horse serum and penicillin/streptomycin at 37 °C with 5% CO2. Cells were plated in 35-mm dishes and transiently transfected with the expression construct for RDH10 in pCMV-HA or pCMV-Tag4a vector using Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen). Twenty-four hours after transfection, growth medium was replaced by a fresh medium containing either 2 μm all-trans-retinol or 5 μm all-trans-retinaldehyde. Retinoids were added to the medium from ethanol stocks. The final ethanol concentration did not exceed 0.6% (v/v). Cells were incubated with retinaldehyde or retinol as indicated. After the incubations, media and cells were collected separately under reduced light and aliquots of cell suspensions were taken for protein quantification and Western blot analysis. Retinoids were extracted into hexane (24) and separated by normal phase HPLC using the Spherisorb S3W column as described above except that the linear mobile phase consisted of 99.6:0.3:0.1 hexane:isopropyl alcohol:acetic acid. Peaks were identified by comparison to retention times of retinoid standards and evaluation of wavelength maxima and quantified as described previously (18, 19).
Western Blot Analysis—The protocol for Western blot analysis was described elsewhere (18). RDH10-His6 expression in Sf9 cells was detected using His tag monoclonal mouse antibody (Novagen, Madison, WI) at a 1:6,000 dilution. HA-RDH10 expression in HEK293 cells was detected using HA tag monoclonal antibody 12CA5 at a 1:100 dilution (antibodies were obtained as a kind gift from Dr. Hengbin Wang, Department of Biochemistry, University of Alabama School of Medicine). Secondary goat anti-mouse horseradish peroxidase-conjugated antibody (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) was used at a 1:40,000 dilution.
RDH10 polyclonal antiserum was generated in rabbit at Cocalico Biologicals (Reamstown, PA). SacI-XhoI fragment encoding amino acids 159–341 was excised from RDH10 pVL1393 expression construct and subcloned into SacI-XhoI sites of pET21(a) vector (Novagen). Truncated RDH10 polypeptide fused to the C-terminal His6 tag was expressed in bacterial cells. The recombinant protein was abundant in the insoluble fraction but exhibited poor binding to metal affinity resin. The insoluble fraction of bacterial homogenate was dissolved in 6 m urea and subjected to electrophoreses in 10% SDS-PAGE. The band corresponding to partial RDH10 was purified by electroelution and used as an antigen. A 1:5,000 dilution of the antiserum detected 2 ng of purified truncated RDH10 when used with a 1:40,000 dilution of peroxidase-conjugated AffiniPure goat anti-rabbit secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) and Pierce ECL Western blotting substrate (Pierce).
RT-PCR Analysis of SDR Gene Expression—To test for endogenous expression of RDH10 in HEK293 cells, total RNA was isolated from HEK293 cells using the RNeasy Mini kit (Qiagen Inc., Valencia, CA). Reverse transcription was performed with random hexamer primers and avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI). A 393-base pair fragment of the RDH10 transcript was amplified using forward primer 5′-AGG GAG AAC GTC TAC CTG AC-3′ and reverse primer 5′-TCT GAA CAT GCC AGT GTC TAC-3′.
RT-PCR analysis of RoDH4 and RL-HSD expression was carried out using gene-specific pairs of primers: 5′-GAC TTC GTG ACC ATA CTG GAC-3′ (forward) and 5′-AAG GTG GGC ATG TAG CTC ATG-3′ (reverse), for RoDH4; and 5′-CAT TCT TAC ACC AAT TAC CTT ATG-3′ (forward) and 5′-GTC ATG TTT GTC ATT CCC GTT C-3′ (reverse) for RL-HSD. The expected sizes of the products for RoDH4 and RL-HSD were 515 and 309 bp, respectively.
siRNA-mediated Knockdowns of SDR Gene Expression—To silence the expression of SDRs in HEK293 cells, the cells were grown in 35-mm dishes to 100% confluence and transfected with corresponding siRNA reagents in OptiMEM using Lipofectamine and Plus reagents (Invitrogen) according to the manufacturer's protocol. Negative control siRNA was ON-TARGETplus™ siCONTROL® pool of four nontargeting duplexes (D-001206-13-20, Dharmacon). The final concentrations of SDR-targeting or control siRNAs were 100 nm. To achieve more efficient silencing, transfections were repeated after a 24-h interval.
Expression of RDH10 was silenced using ON-TARGETplus pool of four RDH10-targeting siRNA duplexes (LU-010008-01-0002, Dharmacon, Inc. Lafayette, CO) or individual siRNA duplexes. Sequences of the individual duplexes were as follows: duplex 9, 5′-CGG CUG AAA GAG UCC GCA AUU-3′; duplex 10, 5′-AGU GUA AAU UAA CCG ACU AUU-3′; duplex 11, 5′-GUU CAG UAC UGC CGG AGU UUU-3′, duplex 12, 5′-CAG UUG ACA UCU UCG CAA UUU-3′. Silencing of RoDH4 and RL-HSD gene expression was carried out using siGENOME SMARTpools M-008311-00 and M-009280-00 (Dharmacon), respectively. One day after the second transfection cells were treated with 10 μm retinol for 24 h and retinoid metabolites were analyzed as described above.
RNA Isolation and Northern Blot Analysis—Total RNA was isolated from siRNA-transfected HEK293 cells using TRIzol reagent (Invitrogen). RNA (20–30 μg) was subjected to electrophoresis in a 1.2% agarose gel containing 2.2 m formaldehyde and transferred to Hybond-XL nylon membrane (Amersham Biosciences). The membrane was hybridized with a 32P-labeled RDH10 549-base pair cDNA probe (SacI-NotI fragment of RDH10-pVL1393 construct) in ExpressHyb hybridization solution (Clontech, Mountain View, CA), according to the manufacturer's instructions. The mRNA bands were visualized by exposure to x-ray film at -80 °C with two intensifying screens for several days. A cDNA probe for glyceraldehyde-3-phosphate dehydrogenase was used as a loading control.
Cycloheximide Treatment—HEK293 cells were plated in 35-mm dishes and transfected with RDH10 in pCMV-Tag4A vector encoding untagged native RDH10 as described above. Twenty-four hours after transfection, medium was replaced with a growth medium containing cycloheximide at 25 μg/ml (added from a 25 mg/ml stock solution in ethanol) or a vehicle. Cells from the cycloheximide-treated and control dishes were harvested 36 h after treatment and the levels of RDH10 protein in homogenates were analyzed by Western blotting using RDH10 rabbit polyclonal antiserum.
Densitometric and Statistical Analysis—Densitometric analysis of the protein bands was done using UN-SCAN-IT software (Silk Scientific Corporation, Orem, UT). Statistical significance was determined by a two-tailed unpaired Student's t test using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). p < 0.05 was considered statistically significant.
RESULTS
Expression and Kinetic Characterization of Human RDH10—To characterize the catalytic properties of human RDH10, we expressed the enzyme in insect Sf9 cells using the Baculovirus expression system (18–20). Recombinant RDH10 protein was abundant in the microsomal fraction of the cells and could be readily visualized by staining with Coomassie Brilliant Blue R-250 after separation of proteins in SDS-PAGE (Fig. 1A). RDH10 constituted ∼10% of the total microsomal protein as determined by gel scanning densitometry. Electrophoretic mobility of the 354-amino acid recombinant His6-tagged RDH10 correlated well with the predicted molecular mass of 39.6 kDa. The activity of RDH10 in the microsomes was relatively stable, but some loss of activity occurred after repetitive freeze-thaw cycles. Purification of RDH10-His6 from Sf9 cells using nickel affinity chromatography produced an inactive enzyme; therefore, microsomal preparations of RDH10 were used for its kinetic characterization as described previously for other microsomal retinoid oxidoreductases (18–20).
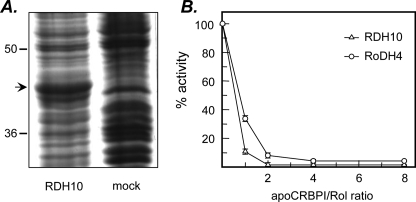
FIGURE 1.
Expression of RDH10 in Sf9 cells and effect of apoCRBPI on RDH10 retinol dehydrogenase activity. A, Sf9 cells were infected with recombinant (RDH10-His6) or wild-type (mock) Baculovirus vector and microsomes were isolated by differential centrifugation 3 days later. Microsomal proteins were separated by SDS-PAGE and the proteins were visualized by staining with Coomassie R-250. The position of RDH10 protein is indicated by an arrow. B, the reactions were carried out with microsomal preparation of RDH10 expressed in Sf9 cells (5 μg) in the presence of 1 mm NAD+ for 15 min at 37 °C. RoDH4 expressed in microsomes of Sf9 cells (5 μg) was used for comparison. The concentration of all-trans-retinol (Rol) was 1μm, whereas the concentration of apoCRBPI was varied from 0 to 8 μm. The results shown are mean ± S.D. of three independent experiments and were reproduced with three different preparations of apoCRBPI.
Specific activity of RDH10 (nmol min-1 mg-1) was the same with 2 or 20 μg of microsomal protein, indicating a linear relationship between the amount of protein and the reaction rate. To determine the cofactor preference of RDH10, the retinol dehydrogenase activity of RDH10-containing microsomes was assayed in the presence of 1 mm NAD+ or NADP+. These assays showed that with NAD+ as a cofactor, RDH10-containing microsomes exhibited a 49-fold greater rate of retinol oxidation than wild-type Sf9 microsomes (1.48 ± 0.2 versus 0.03 ± 0.01 nmol min-1 mg-1). In contrast, with NADP+ as a cofactor, the activity of RDH10-containing microsomes did not exceed that of wild-type microsomes (0.17 ± 0.03 nmol min-1 mg-1). Further analysis of RDH10 activity over a wide range of NADP+ concentrations (2–6,250 μm) using 2–20 μg of microsomal protein confirmed that RDH10 was not active in the presence of NADP+. Thus, RDH10 appeared to be strictly an NAD+-dependent oxidoreductase, contrary to the previous report (16).
Kinetic analysis revealed that the apparent Km value of RDH10 for NAD+ with all-trans-retinol as substrate was ∼100 μm (Table 1). RDH10 was also quite active in the reductive direction with all-trans-retinaldehyde in the presence of NADH as a cofactor, challenging earlier findings (16), which showed little or no activity of RDH10 toward all-trans-retinaldehyde. In fact, the apparent Vmax value of RDH10 in the reductive direction was higher than that in the oxidative direction, but because of the ∼10-fold lower Km value for all-trans-retinol, the catalytic efficiency of RDH10 (Vmax/Km) was greater in the oxidative direction (Table 1), indicating that RDH10 is a more efficient retinol dehydrogenase than a retinaldehyde reductase.
TABLE 1.
Kinetic constants of human RDH10 Kinetic constants were determined using the same preparation of microsomes containing RDH10. Similar constants were obtained using three independent preparations of microsomal RDH10.
| Substrate | Apparent Km | Apparent Vmax | Vmax/Km |
|---|---|---|---|
| μm | nmol min–1 mg–1 | ||
| NAD+ | 100 ± 10 | 1.30 ± 0.04 | |
| All-trans-retinol | 0.035 ± 0.010 | 1.30 ± 0.05 | 37 |
| All-trans-retinaldehyde | 0.4 ± 0.1 | 2.7 ± 0.2 | 6.8 |
| 11-cis-Retinol | 0.06 ± 0.01 | 1.42 ± 0.06 | 24 |
| 9-cis-Retinol | 0.04 ± 0.01 | 0.77 ± 0.05 | 19 |
Also in contradiction with the previous report (16), which suggested that RDH10 was inactive toward cis-retinols, our data showed that RDH10 recognized 11-cis-retinol and 9-cis-retinol as substrates with the apparent Km and Vmax values very similar to those for all-trans-retinol (Table 1). The catalytic efficiency of RDH10 appeared to be somewhat greater for all-trans-retinol, but it was closely followed by that for 11-cis-retinol or 9-cis-retinol (Table 1). For all three retinols there was an apparent substrate inhibition at higher concentrations (>1 μm for cis-retinols and >5 μm for all-trans-retinol).
Previous studies indicated that RDH10 is expressed in liver cells, in retinal pigment epithelial cells, and in Müller cells of the eye (16, 25). All of these cells contain CRBPI (25–27). CRBPI was shown to sequester all-trans-retinol from human microsomal retinol dehydrogenases, RoDH4 and RL-HSD (21, 28). To determine whether RDH10 can oxidize all-trans-retinol in the presence of CRBPI, the activity of the enzyme was assayed at different molar ratios of apoCRBPI to retinol. At a 1:1 ratio, the amount of retinaldehyde produced by RDH10 from 1 μm retinol constituted 34% of the amount produced in the absence of apoCRBPI (Fig. 1B). In comparison, under the same conditions, human microsomal RoDH4 produced only 10% of retinaldehyde from that in the absence of apoCRBPI (Fig. 1B). For each enzyme, the amount of retinaldehyde produced from retinol decreased with increasing concentrations of apoCRBPI, although the amount of retinaldehyde generated by RDH10 was always greater compared with RoDH4.
To determine whether RDH10 could utilize retinol bound to CRBPI as a substrate, we saturated CRBPI with all-trans-retinol and purified holoCRBPI from unbound retinol using size-exclusion column chromatography as described previously (28). At 5 μm retinol, the reaction rate toward holoCRBPI was significantly lower (0.08 nmol min-1 mg-1) than that toward free retinol (1.5 nmol min-1 mg-1). This dramatic difference in the activity with free versus bound retinol was similar to that observed for other retinoid active oxidoreductases (18, 19, 21, 28), suggesting that RDH10 also recognizes only the free form of retinol as a substrate.
Analysis of RDH10 Activity toward Retinol and Retinaldehyde in Living Cells—Because RDH10 was found to exhibit a greater rate in the reductive than in the oxidative direction in vitro, we examined the direction of the RDH10-catalyzed reaction in living cells. RDH10 was overexpressed in HEK293 cells and the cells were incubated with all-trans-retinol or all-trans-retinaldehyde. Analysis of RDH10 activity was carried out in triplicates 24 h after transfection. Immunoblotting showed that the levels of RDH10 protein in parallel samples were very similar (Fig. 2A). With all-trans-retinol as a substrate, there was a significant increase in the level of all-trans-retinaldehyde produced by RDH10-transfected cells (0.32 ± 0.1 nmol mg-1) as compared with mock-transfected cells (0.01 ± 0.01 nmol mg-1) (Fig. 2B). The increase in retinaldehyde was accompanied by a ∼14-fold increase in the level of retinoic acid (0.41 ± 0.07 versus 0.03 ± 0.01 nmol mg-1) (Fig. 2B, a representative HPLC chromatogram is shown in supplemental Fig. S1). Thus, RDH10 exhibited an oxidative activity, converting retinol to retinaldehyde and contributing to retinoic acid biosynthesis.
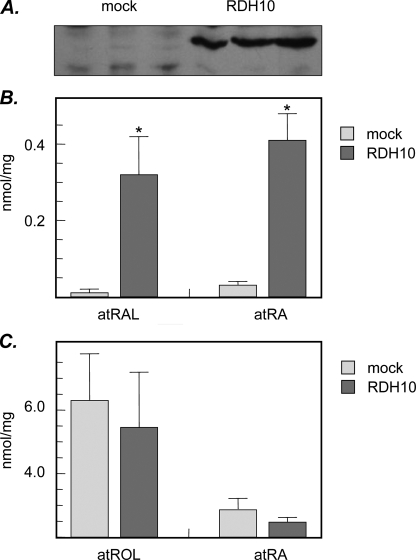
FIGURE 2.
Increase in retinoic acid biosynthesis in RDH10-transfected cells. A, HEK293 cells were transfected with HA-RDH10/pCMV-HA vector (RDH10) or empty vector (mock). Expression of RDH10 was confirmed by Western blotting using HA antibodies. Cells were incubated with all-trans-retinol (2 μm) for 6 h (B) or with all-trans-retinaldehyde (5 μm) for 4 h (C). Retinoid metabolites were analyzed by normal phase HPLC. atROL, all-trans-retinol; atRAL, all-trans-retinaldehyde; atRA, all-trans-retinoic acid. Data are mean ± S.D. (vertical bars), n = 3. Statistical analysis was done using two-tailed unpaired Student's t test comparing RDH12-transfected cells to mock-transfected cells; *, p < 0.001. The results are representative of three independent experiments.
Importantly, when retinaldehyde was provided to the cells as a substrate, there was no difference in the levels of retinol or retinoic acid generated from retinaldehyde in RDH10-transfected cells versus mock-transfected cells (Fig. 2C), indicating that RDH10 did not contribute to the reduction of retinaldehyde. These results indicated that although bidirectional in vitro, RDH10 functioned exclusively in the oxidative direction in the cells. Notably, the amount of retinoic acid produced by the cells from retinaldehyde was severalfold greater than that produced from retinol, consistent with the oxidation of retinol being the rate-limiting step in retinoic acid biosynthesis (2).
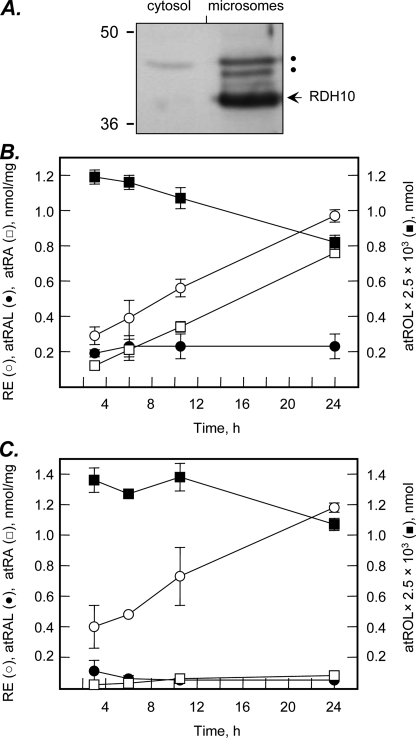
To gain further insight into the kinetics of retinol utilization by RDH10 in HEK293 cells, we determined the time course of retinol metabolism in RDH10-transfected versus mock-transfected cells. The cells were transfected with untagged RDH10 construct and RDH10 protein association with the microsomal membranes was confirmed by immunoblotting using RDH10 polyclonal antiserum (Fig. 3A). Analysis of retinol metabolites showed that the amount of retinoic acid produced by RDH10-transfected cells increased linearly in time at a rate that was ∼10-fold greater than that in mock-transfected cells (Fig. 3, B and C). At the same time, the level of retinaldehyde, the intermediate product in the conversion of retinol to retinoic acid, remained constant after the initial burst, in agreement with the steady-state conditions. Retinol was also converted to retinyl esters in both RDH10-transfected and mock-transfected cells. Interestingly, the amount of retinyl esters in RDH10-transfected cells was consistently lower then in mock-transfected cells, although this difference reached statistical significance only by 24 h (1.2 ± 0.03 versus 1 ± 0.03 nmol mg-1, p = 0.001). This observation suggested that RDH10 was able to compete with retinol esterases for retinol.
FIGURE 3.
Time course of retinol metabolism in HEK293 cells. A, HEK293 cells were transfected with untagged RDH10 expression construct in pCMV-Tag4a and the microsomes and cytosol were isolated by differential centrifugation. RDH10 protein was detected by Western blotting using RDH10 rabbit polyclonal antiserum. The slower moving bands indicated by dots are due to nonspecific binding of antibodies and are not glycosylated forms of RDH10, because RDH10 polypeptide sequence does not contain any consensus N-glycosylation sites (NxS/T). B, RDH10-transfected cells were incubated with all-trans-retinol (2 μm) for the indicated periods of time. C, cells transfected with empty vector served as controls for endogenous cellular activities. Retinoids were extracted and analyzed by normal phase HPLC as described under “Experimental Procedures.” RE, retinyl esters; other abbreviations are as described in the legend to Fig. 2.
siRNA-mediated Down-regulation of Endogenous RDH10 Expression—Because untransfected HEK293 cells had some baseline level of retinol dehydrogenase activity, we examined whether RDH10 was present in these cells. RT-PCR analysis using RDH10-specific oligonucleotide primers showed that HEK293 cells do, in fact, express RDH10 mRNA (data not shown). To examine the contribution of endogenous RDH10 to retinoic acid biosynthesis, we knocked down RDH10 expression using a pool of four RDH10-specific siRNA duplexes from Dharmacon Inc. (Lafayette, CO). The levels of endogenous RDH10 protein in the microsomal fractions of HEK293 cells or other cultured cells such as MCF10A, LNCaP, or SK-N-SH proved to be below the detection limit (∼2 ng) of RDH10 rabbit polyclonal antiserum. Therefore, the efficiency of RDH10 gene expression knockdown was monitored by Northern blot analysis. This analysis showed that the treatment of cells with a pool of anti-RDH10 siRNAs decreased the amount of RDH10 mRNA to undetectable levels (Fig. 4A). This decrease in RDH10 mRNA was accompanied by a ∼2-fold decrease in retinoic acid biosynthesis compared with cells transfected with non-targeting siRNAs (100 ± 8 versus 47 ± 3%, p = 0.0004) (Fig. 4B). To confirm the specificity of RDH10 contribution, we used individual RDH10-specific siRNA duplexes. Treatment with duplex 10 was the most effective and resulted in a 1.7-fold decrease in retinoic acid production from retinol (100 ± 13 versus 58 ± 12% in control cells, p = 0.015) (Fig. 4C). Thus, RDH10 was proven to contribute to endogenous retinol oxidation for retinoic acid biosynthesis in the cells.
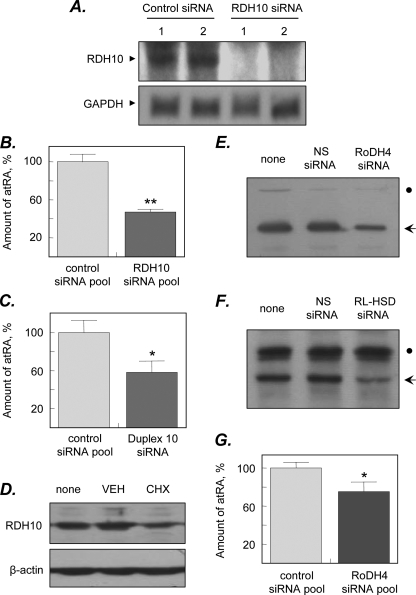
FIGURE 4.
Effect of siRNA-mediated knockdown of SDRs on retinoic acid biosynthesis. A, HEK293 cells were transfected with ON-TARGETplus pool of four siRNAs targeted against RDH10 or siCONTROL Non-targeting siRNA pool. Knockdown of RDH10 mRNA expression (RDH10) was confirmed by Northern blot analysis. A cDNA probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Shown are the results of two parallel experiments (1 and 2). B, cells were incubated with 10 μm all-trans-retinol for 24 h and all-trans-retinoic acid (atRA) levels were analyzed by HPLC. Data shown are average ± S.D. of triplicates from one of three experiments; **, p = 0.0004. C, HEK293 cells were transfected with RDH10 duplex 10 (J-01008-10, Dharmacon) or siCONTROL. Non-targeting siRNA pool and the levels of retinoic acid were determined by normal phase HPLC. Data shown are average ± S.D. of triplicates from one of two experiments; *, p = 0.015. D, untagged RDH10 was expressed in HEK293 cells and the cells were treated with either vehicle (VEH) or cycloheximide (CHX) for 36 h. The levels of RDH10 protein in the cells before (none) and after treatments were determined by immunoblotting with RDH10 polyclonal antiserum. Staining with β-actin antibodies was used as a control for loading of samples. E and F, HEK293 cells stably expressing RoDH4 (E) or RL-HSD (F) protein were transfected with the siGENOME SMARTpools M-008311-00 and M-009280-00 (Dharmacon), respectively, and the levels of corresponding proteins were determined by Western blot analysis 3 days after transfections. The positions of RoDH4 and RL-HSD are indicated by arrows. Dots indicate nonspecific staining, which attests to equal loading of the samples. G, cells transfected with RoDH4-specific siRNA pool or control siRNA were incubated with 10 μm retinol for 24 h and the levels of retinoids were analyzed by HPLC as described in B. Data shown are average ± S.D. of triplicates from one of three experiments; *, p = 0.02.
The remaining retinol dehydrogenase activity that supported ∼50% of retinoic acid biosynthesis after silencing of RDH10 mRNA expression could be due to the presence of additional retinol dehydrogenases or residual levels of RDH10 protein. To determine whether the RDH10 protein remained in the cells after silencing of mRNA expression, we examined longevity of the RDH10 protein in HEK293 cells. The native untagged form of RDH10 was overexpressed in the cells to enable the detection of RDH10 protein by Western blotting and the cells were treated with the inhibitor of protein synthesis cycloheximide or with a vehicle. As shown in Fig. 4D, ∼50% of RDH10 protein remained in the cells 36 h after cycloheximide treatment, suggesting that RDH10 has a relatively long half-life. Thus, some of the retinoic acid biosynthesis observed in the cells 2 days after the addition of RDH10 siRNAs could be due to residual levels of the long-lived RDH10 protein.
To explore the possibility that other enzymes are partly responsible for retinoic acid biosynthesis in HEK293 cells, we examined the contribution of two human NAD+-dependent SDRs, RoDH4 and RL-HSD, which were previously shown to oxidize retinol when overexpressed in the cells (21)3 and RT-PCR analysis revealed the presence of RoDH4 mRNA in HEK293 cells, but suggested only trace levels of RL-HSD mRNA (data not shown). The corresponding proteins could not be detected in HEK293 cells by immunoblotting using available antisera (20, 29), therefore, the efficiency of siRNA pools specific for RoDH4 and RL-HSD was tested using stably transfected cell lines overexpressing RoDH4 and RL-HSD (21, 30). Both siRNA pools decreased the levels of their respective proteins by >50% after 3 days of incubation (Fig. 4, E and F), consistent with targeting of their corresponding mRNAs.
Treatment of wild-type HEK293 cells with the RL-HSD siRNA pool to assess the contribution of endogenously expressed RL-HSD did not result in a decrease of retinoic acid biosynthesis. However, treatment of the cells with the RoDH4 siRNA pool decreased retinoic acid production from retinol by approximately ∼25% (100 ± 6 versus 75 ± 10%, p = 0.02) (Fig. 4G). This result indicated that, like RDH10, human RoDH4 contributes to endogenous retinol oxidation in HEK293 cells and may be at least in part responsible for the remaining retinoic acid production in the cells treated with RDH10 siRNA. Simultaneous treatment of the cells with both RDH10 and RoDH4 siRNAs had an additive effect on retinoic acid production, consistent with targeting of two independent enzymes (data not shown).
DISCUSSION
RDH10 is most closely related to one of the least characterized groups of SDRs that includes retinal SDR1 (31), 17β-HSD11 (32), epidermal retinal dehydrogenase 2 (RDHE2) (33), and 17β-HSD13 (34). The earlier study implicated RDH10 in the oxidative metabolism of retinol to retinaldehyde, because when expressed in the cells, RDH10 appeared to increase the levels of retinoic acid (16). Surprisingly, analysis of RDH10 properties showed that RDH10 prefers NADP+ as a cofactor (16), suggesting a completely different physiological function for RDH10 as a reductive enzyme in the formation of storage forms of retinoids, retinol and retinyl esters, rather than as an oxidative enzyme in the pathway of retinoic acid biosynthesis. This contradiction between the cofactor specificity of the enzyme and behavior in the cells prompted us to re-investigate the properties of RDH10.
The results of our study demonstrate that RDH10 is a strictly NAD+-dependent SDR. RDH10 expressed in the microsomes of Sf9 cells exhibits significant retinol dehydrogenase or retinaldehyde reductase activity with NAD+ or NADH as cofactors, but has no detectable activity in the presence of NADP+/NADPH. In most cells the concentration of oxidized NAD+ greatly exceeds that of NADH (35–38), suggesting that the NAD(H)-preferring enzymes will function in the oxidative direction. Indeed, as shown in this study, although RDH10 is bidirectional in vitro, in the cells, the enzyme acts unidirectionally in the oxidative direction, increasing the levels of retinaldehyde and retinoic acid. This is in contrast to the behavior of the NADP+-dependent enzyme, RDH12 (39). In vitro, RDH12 oxidizes retinol at a ∼60-fold greater rate than RDH10 (Table 2). However, when expressed in HEK293 cells, RDH12 functions exclusively in the reductive direction, increasing the amount of retinol and retinyl esters but reducing the level of retinoic acid (39). This is consistent with the data showing that the concentration of NADPH in the cells is usually greater than that of NADP+ (35–38). Because otherwise similar enzymes, RDH10 and RDH12, function in opposite directions in the same cells, it is clear that the direction of their reactions is determined by their respective cofactor preferences. Interestingly, the Km value of RDH10 for NAD+ is significantly higher than those of other retinoid-active SDRs (Table 2). The reported cellular concentrations of NAD+ are in the range of ∼150–300 μm (35–38), suggesting that the factors that influence the NAD+/NADH ratio in the cells, e.g. oxidative stress, may influence cellular RDH10 retinol dehydrogenase activity and, as a result, retinoic acid biosynthesis.
TABLE 2.
Comparison of RDH10 with other microsomal retinoid oxidoreductases All enzymes were expressed in Sf9cells. Expression levels of respective proteins were comparable.
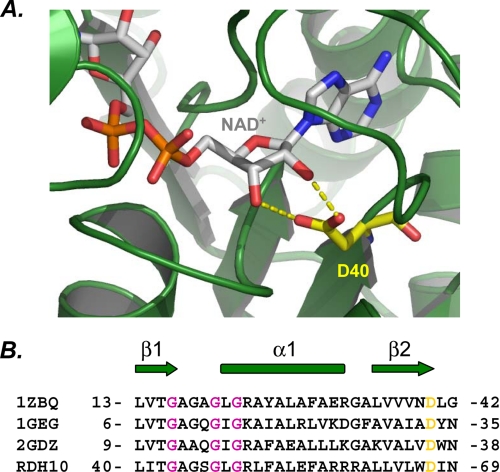
Our finding that RDH10 prefers NAD+ as a cofactor is consistent with the current understanding of the structural determinants of SDR cofactor specificity (40). Structure-function analysis of SDRs strongly suggests that their cofactor specificity is determined by the presence of the aspartate residue in the βαβ motif at the beginning of the Rossmann fold (40, 41) (Fig. 5). This residue directly contributes to NAD+ binding by forming two hydrogen bonds with the 2′ and 3′ hydroxyl groups of ribose (Fig. 5A). At the same time, aspartate takes up the space that would be occupied by the phosphoryl group of NADP+, thereby creating sterical hindrance and a charge repulsion mechanism for the exclusion of NADP+. Alignment of the cofactor-binding motif of RDH10 with those of other NAD+-preferring SDRs clearly shows that the corresponding position in RDH10 is occupied by Asp-67 (Fig. 5B), indicating that RDH10 is an NAD(H)-preferring enzyme, in agreement with our experimental data.
FIGURE 5.
Cofactor specificity of RDH10. A, ribbon representation of the dehydrogenase domain of human 17β-HSD4 (PDB code 1ZBQ). Shown is the view of the cofactor binding region of the Rossmann fold with bound NAD+. NAD+ and D40 are shown as stick models (CPK, carbon gray and CPK, carbon yellow, respectively). Hydrogen bonds between Asp-40 and NAD+ are shown in yellow. Molecular graphics for structural representation were made using PyMol (DeLano Scientific LLC, Palo Alto, CA). B, structural alignment of NAD+-dependent SDRs. The secondary structure elements are indicated as arrows for β-strands and as cylinders for α-helices. Conserved aspartate residues that determine the preference for NAD+ are highlighted in yellow. Gly residues that constitute the GXXXGXG cofactor-binding motif are shown in dark pink. 1GEG, crystal structure of meso-2,3-butanediol dehydrogenase in a complex with NAD+ and inhibitor mercaptoethanol; 2GDZ, crystal structure of 15-hydroxyprostaglandin dehydrogenase type 1, complexed with NAD+.
An important finding of this study is that RDH10 has the highest affinity for all-trans-retinol among all SDR and ADH retinoid oxidoreductases. For example, the affinities of two other human SDR enzymes that function in the oxidative direction, RoDH4 and RL-HSD, are at least 28-fold lower than that of RDH10 (Table 2). This unique property allows RDH10 to effectively compete with CRBPI for all-trans-retinol. As a result, RDH10 maintains a significant rate of retinol oxidation in the presence of varied concentrations of CRBPI. In agreement with this observation, silencing of RDH10 expression in HEK293 cells, which contain CRBPI (23), results in a decrease in retinoic acid biosynthesis. This distinguishes RDH10 from other enzymes that also recognize only the free form of retinol but are less able to compete with CRBPI (8, 18, 19, 21, 28).
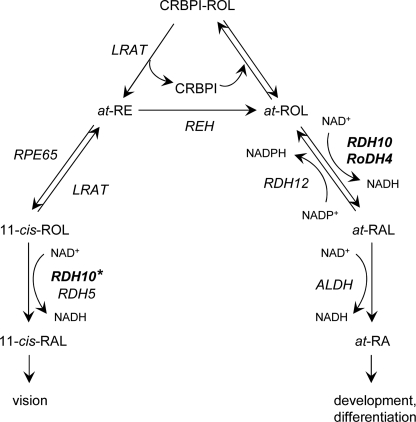
Retinol is the source of bioactive retinoids that serve two principally different physiological functions: all-trans-retinoic acid serves as a mediator during differentiation and development, whereas 11-cis-retinaldehyde is the chromophore required for vision. As shown in Fig. 6, all-trans-retinol is converted to all-trans-retinoic acid and 11-cis-retinaldehyde through two different metabolic pathways. All-trans-retinoic acid is produced from all-trans-retinol in two oxidative steps: oxidation of retinol to retinaldehyde followed by oxidation of retinaldehyde to retinoic acid. The first step is rate-limiting and reversible (2). Increasing evidence indicates that this reversibility is due to the presence of both NAD+ and NADP+-dependent retinoid oxidoreductases in the same cells. Thus, the overall rate of retinoic acid biosynthesis may be regulated by the ratio of oxidative and reductive enzymes.
FIGURE 6.
Schematic of retinoid metabolic pathways. The schematic illustrates the role of RDH10 in the biosynthesis of all-trans-retinoic acid for development and differentiation and in the biosynthesis of 11-cis-retinaldehyde for vision. The 11-cis-retinol dehydrogenase activity of RDH10 is marked with a star to indicate that this activity has not yet been demonstrated in vivo. REH, retinyl ester hydrolase; RPE65, isomerohydrolase; ALDH, aldehyde dehydrogenase. Other abbreviations are as described in the legend to Fig. 2. Note that the NAD+-dependent RDH10 and RoDH4 function in the oxidative direction, whereas the NADP+-dependent RDH12 functions in the reductive direction. Lecithin:retinol acyltransferase (LRAT) appears to utilize all-trans-retinol bound to CRBPI, whereas the oxidoreductases exclusively recognize the unbound all-trans-retinol as a substrate.
CRBPI channels retinol toward esterification by lecithin:retinol acyltransferase, which was shown to recognize the bound form of retinol as a substrate (42). Thus, CRBPI appears to function as a switch that regulates the availability of retinol for oxidation versus storage (Fig. 6). Interestingly, the expression levels of CRBPI are increased by retinoic acid (43–49). This suggests a feedback regulation loop for retinoic acid biosynthesis by CRBPI. An increase in retinoic acid would increase the levels of CRBPI, shifting the equilibrium toward the bound form of retinol and increasing the availability of holoCRBPI for lecithin:retinol acyltransferase while reducing the biosynthesis of retinoic acid (Fig. 6). Notably, mice that lack CRBPI have a faster turnover rate of retinol (50, 51), consistent with the greater rate of retinol oxidation in the absence of CRBPI.
11-cis-Retinaldehyde is derived from all-trans-retinol through a series of enzymatic reactions that involves the conversion of CRBPI-bound retinol to all-trans-retinyl esters catalyzed by lecithin:retinol acyltransferase, followed by isomerization and hydrolysis of retinyl esters to 11-cis-retinol catalyzed by isomerohydrolase RPE65 (52) (Fig. 6). 11-cis-Retinol is then oxidized to 11-cis-retinaldehyde, and this reaction is believed to be catalyzed by at least two enzymes, RDH5 and RDH11 (15, 53).
In this study, we show that RDH10 is a multisubstrate enzyme that recognizes both all-trans- and 11-cis-retinol. This suggests that RDH10 can contribute to both pathways of retinoid metabolism. As an oxidative enzyme, RDH10 increases the rate of all-trans-retinaldehyde production from all-trans-retinol, which is then oxidized irreversibly to retinoic acid by aldehyde dehydrogenases (Fig. 6). Hence, RDH10 activity should have a direct impact on the levels of retinoic acid and, as a result, on differentiation and development of tissues. This conclusion is supported by the previous report that mice carrying the inactivating mutation in the Rdh10 gene do not survive past day 13 of embryogenesis (17).
The role of RDH10 in biosynthesis of 11-cis-retinaldehyde is supported by observations that patients harboring null mutations in RDH5 ultimately recover their visual pigments, suggesting that there are additional enzymes that oxidize 11-cis-retinol in the eye. Furthermore, rdh5-/-rdh11-/- double knock-out mice have a relatively mild visual phenotype (53), providing further evidence for the existence of additional 11-cis-retinol dehydrogenases. As shown in this study, RDH10 is well suited for this role, because it exhibits exceptionally high affinity for 11-cis-retinol, prefers NAD+ as a cofactor, and functions in the oxidative direction in the cells.
Supplementary Material
Acknowledgments
We thank Dr. Hengbin Wang (University of Alabama School of Medicine, Birmingham, AL) for antibodies against HA tag, and Dr. Rosalie Crouch (Medical University of South Carolina, Charleston, SC) for 11-cis-retinaldehyde.
This work was supported, in whole or in part, by National Institutes of Health Grant AA12153 from the NIAAA (to N. Y. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: ADH, alcohol dehydrogenase; RDH or RoDH, retinol dehydrogenase; HSD, hydroxysteroid dehydrogenase; RL-HSD, RoDH-like 3α-hydroxysteroid dehydrogenase; SDR, short-chain dehydrogenase/reductase; CRBP, cellular retinol binding protein; HEK293 cells, human embryonic kidney cells; HA, hemagglutinin; HPLC, high-pressure liquid chromatography; RT, reverse transcriptase; siRNA, small interfering RNA.
E. V. Shabrova and N. Y. Kedishvili, unpublished observations.
References
- 1.Mangelsdorf, D., Umesono, K., and Evans, R. M. (1994) in The Retinoids: Biology, Chemistry and Medicine (Sporn, M. B., Roberts, A. B., and Goodman, D. S., eds) pp. 319-350, Raven Press, New York
- 2.Napoli, J. L. (1999) Biochim. Biophys. Acta 1440 139-162 [DOI] [PubMed] [Google Scholar]
- 3.Duester, G. (1996) Biochemistry 35 12221-12227 [DOI] [PubMed] [Google Scholar]
- 4.Kedishvili, N. Y. (2002) Curr. Org. Chem. 6 1247-1257 [Google Scholar]
- 5.Veech, R. L., Eggleston, L. V., and Krebs, H. A. (1969) Biochem. J. 115 609-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerman, M. H., and Napoli, J. L. (1995) Biochemistry 34 7027-7037 [DOI] [PubMed] [Google Scholar]
- 7.Belyaeva, O. V., and Kedishvili, N. Y. (2006) Genomics 88 820-830 [DOI] [PubMed] [Google Scholar]
- 8.Kedishvili, N. Y., Gough, W. H., Davis, W. I., Parsons, S., Li, T. K., and Bosron, W. F. (1998) Biochem. Biophys. Res. Commun. 249 191-196 [DOI] [PubMed] [Google Scholar]
- 9.Deltour, L., Foglio, M. H., and Duester, G. (1999) J. Biol. Chem. 274 16796-16801 [DOI] [PubMed] [Google Scholar]
- 10.Molotkov, A., Deltour, L., Foglio, M. H., Cuenca, A. E., and Duester, G. (2002) J. Biol. Chem. 277 13804-13811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molotkov, A., Fan, X., Deltour, L., Foglio, M. H., Martras, S., Farrés, J., Parés, X., and Duester, G. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 5337-5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, M., Hu, P., Krois, C. R., Kane, M. A., and Napoli, J. L. (2007) FASEB J. 21 2886-2896 [DOI] [PubMed] [Google Scholar]
- 13.Hu, P., Zhang, M., and Napoli, J. L. (2007) Biochim. Biophys. Acta 1770 694-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon, A., Hellman, U., Wernstedt, C., and Eriksson, U. (1995) J. Biol. Chem. 270 1107-1112 [PubMed] [Google Scholar]
- 15.Yamamoto, H., Simon, A., Eriksson, U., Harris, E., Berson, E. L., and Dryja, T. P. (1999) Nat. Genet. 22 188-191 [DOI] [PubMed] [Google Scholar]
- 16.Wu, B. X., Chen, Y., Chen, Y., Fan, J., Rohrer, B., Crouch, R. K., and Ma, J. X. (2002) Investig. Ophthalmol. Vis. Sci. 43 3365-3372 [PubMed] [Google Scholar]
- 17.Sandell, L. L., Sanderson, B. W., Moiseyev, G., Johnson, T., Mushegian, A., Young, K., Rey, J. P., Ma, J. X., Staehling-Hampton, K., and Trainor, P. A. (2007) Genes Dev. 21 1113-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belyaeva, O. V., Stetsenko, A. V., Nelson, P., and Kedishvili, N. Y. (2003) Biochemistry 42 14838-14845 [DOI] [PubMed] [Google Scholar]
- 19.Belyaeva, O. V., Korkina, O. V., Stetsenko, A. V., Kim, T., Nelson, P. S., and Kedishvili, N. Y. (2005) Biochemistry 44 7035-7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gough, W. H., VanOoteghem, S., Sint, T., and Kedishvili, N. Y. (1998) J. Biol. Chem. 273 19778-19785 [DOI] [PubMed] [Google Scholar]
- 21.Gallego, O., Belyaeva, O. V., Porté, S., Ruiz, F. X., Stetsenko, A. V., Shabrova, E. V., Kostereva, N. V., Farrés, J., Parés, X., and Kedishvili, N. Y. (2006) Biochem. J. 399 101-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, Y., Ma, J. X., and Crouch, R. K. (2003) Mol. Vis. 9 345-354 [PubMed] [Google Scholar]
- 23.Chen, Y., Moiseyev, G., Wu, B. X., Ma, J. X., and Crouch, R. K. (2003) Vision Res. 43 3037-3044 [DOI] [PubMed] [Google Scholar]
- 24.Napoli, J. L., and Horst, R. L. (1998) in Methods in Molecular Biology: Retinoid Protocols (Redfern, C. P. F., ed) Vol. 89, pp. 29-40, Humana Press Inc., Totowa, NJ [DOI] [PubMed] [Google Scholar]
- 25.Wu, B. X., Moiseyev, G., Chen, Y., Rohrer, B., Crouch, R. K., and Ma, J. X. (2004) Investig. Ophthalmol. Vis. Sci. 45 3857-3862 [DOI] [PubMed] [Google Scholar]
- 26.Bok, D., Ong, D. E., and Chytil, F. (1984) Investig. Ophthalmol. Vis. Sci. 25 877-883 [PubMed] [Google Scholar]
- 27.Ong, D. E., and Page, D. L. (1986) Am. J. Clin. Nutr. 44 425-430 [DOI] [PubMed] [Google Scholar]
- 28.Lapshina, E. A., Belyaeva, O. V., Chumakova, O. V., and Kedishvili, N. Y. (2003) Biochemistry 42 776-784 [DOI] [PubMed] [Google Scholar]
- 29.Chetyrkin, S. V., Hu, J., Gough, W. H., Dumaual, N., and Kedishvili, N. Y. (2001) Arch. Biochem. Biophys. 386 1-10 [DOI] [PubMed] [Google Scholar]
- 30.Belyaeva, O. V., Chetyrkin, S. V., Clark, A. L., Kostereva, N. V., SantaCruz, K. S., Chronwall, B. M., and Kedishvili, N. Y. (2007) Endocrinology 148 2148-2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haeseleer, F., Huang, J., Lebioda, L., Saari, J. C., and Palczewski, K. (1998) J. Biol. Chem. 273 21790-21799 [DOI] [PubMed] [Google Scholar]
- 32.Brereton, P., Suzuki, T., Sasano, H., Li, K., Duarte, C., Obeyesekere, V., Haeseleer, F., Palczewski, K., Smith, I., Komesaroff, P., and Krozowski, Z. (2001) Mol. Cell. Endocrinol. 171 111-117 [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaka, Y., Okamoto, K., Tsuji, H., Mabuchi, T., Ozawa, A., Tamiya, G., and Inoko, H. (2002) Biochem. Biophys. Res. Commun. 297 1171-1180 [DOI] [PubMed] [Google Scholar]
- 34.Liu, S., Huang, C., Li, D., Ren, W., Zhang, H., Qi, M., Li, X., and Yu, L. (2007) Acta Biochim. Pol. 54 213-218 [PubMed] [Google Scholar]
- 35.Matschinsky, F. M. (1968) J. Neurochem. 15 643-657 [DOI] [PubMed] [Google Scholar]
- 36.Giblin, F. J., and Reddy, V. N. (1980) Exp. Eye Res. 31 601-609 [DOI] [PubMed] [Google Scholar]
- 37.Bublitz, C., and Lawler, C. A. (1987) Biochem. J. 245 263-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams, J. D., Jr., Klaidman, L. K., Chang, M. L., and Yang, J. (2001) Curr. Top. Med. Chem. 1 473-482 [DOI] [PubMed] [Google Scholar]
- 39.Lee, S. A., Belyaeva, O. V., Popov, I. K., and Kedishvili, N. Y. (2007) J. Biol. Chem. 282 35621-35628 [DOI] [PubMed] [Google Scholar]
- 40.Kallberg, Y., Oppermann, U., Jörnvall, H., and Persson, B. (2002) Eur. J. Biochem. 269 4409-4417 [DOI] [PubMed] [Google Scholar]
- 41.Rossmann, M. G., Liljas, A., Brändén, C.-I., and Banaszak, L. J. (1975) in The Enzymes (Boyer, P. D., ed) Vol. 11, Third Ed., pp. 61-102, Academic Press, New York [Google Scholar]
- 42.Herr, F. M., and Ong, D. E. (1992) Biochemistry 31 6748-6755 [DOI] [PubMed] [Google Scholar]
- 43.Kato, M., Blaner, W. S., Mertz, J. R., Das, K., Kato, K., and Goodman, D. S. (1985) J. Biol. Chem. 260 4832-4838 [PubMed] [Google Scholar]
- 44.Blaner, W. S., Das, K., Mertz, J. R., Das, S. R., and Goodman, D. S. (1986) J. Lipid Res. 10 1084-1088 [PubMed] [Google Scholar]
- 45.Rajan, N., Blaner, W. S., Soprano, D. R., Suhara, A., and Goodman, D. S. (1990) J. Lipid Res. 31 821-829 [PubMed] [Google Scholar]
- 46.Harada, H., Miki, R., Masushige, S., and Kato, S. (1995) Endocrinology 136 5329-5335 [DOI] [PubMed] [Google Scholar]
- 47.Okuno, M., Caraveo, V. E., Goodman, D. S., and Blaner, W. S. (1995) J. Lipid Res. 36 137-147 [PubMed] [Google Scholar]
- 48.Fisher, G. J., Reddy, A. P., Datta, S. C., Kang, S., Yi, J. Y., Chambon, P., and Voorhees, J. J. (1995) J. Investig. Dermatol. 105 80-86 [DOI] [PubMed] [Google Scholar]
- 49.Smith, W. C., Nakshatri, H., Leroy, P., Rees, J., and Chambon, P. (1991) EMBO J. 10 2223-2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matt, N., Schmidt, C. K., Dupé, V., Dennefeld, C., Nau, H., Chambon, P., Mark, M., and Ghyselinck, N. B. (2005) Dev. Dyn. 233 167-176 [DOI] [PubMed] [Google Scholar]
- 51.Molotkov, A., Ghyselinck, N. B., Chambon, P., and Duester, G. (2004) Biochem. J. 383 295-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moiseyev, G., Chen, Y., Takahashi, Y., Wu, B. X., and Ma, J. X. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 12413-12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim, T. S., Maeda, A., Maeda, T., Heinlein, C., Kedishvili, N., Palczewski, K., and Nelson, P. S. (2005) J. Biol. Chem. 280 8694-8704 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.