Abstract
CXCL12/stromal cell-derived factor-1α (SDF-1α), a chemokine ligand for the G protein-coupled receptor CXCR4, plays an important role in the directed movement of cells. Many studies have documented the importance of CXCR4 in tumor progression and organ-specific metastasis. Recently, several studies have implicated a role for SDF-1α in head and neck squamous cell carcinoma (HNSCC) metastasis, but currently there is little information about how SDF-1α promotes HNSCC metastasis. In this report we show that the NF-κB signaling pathway is activated in response to SDF-1α in HNSCC while primary and immortalized keratinocytes show no SDF-1α-mediated NF-κB activity. We found that SDF-1α-mediated NF-κB signaling is independent of phosphoinositide 3-kinase/Akt and ERK/MAPK pathways. We observed that SDF-1α induces IκBα phosphorylation and degradation and the nuclear translocation of NF-κB in HNSCC cell lines, suggesting that SDF-1α activates the classical NF-κB signaling pathway. Contrary to previous reports, SDF-1α-induced NF-κB activation is not mediated by tumor necrosis factor α. Furthermore, blocking the NF-κB signaling pathway with an IKKβ inhibitor significantly reduces SDF-1α-mediated HNSCC invasion. Taken together, our data suggest SDF-1α/CXCR4 may promote HNSCC invasion and metastasis by activating NF-κB and that targeting NF-κB may provide therapeutic opportunities in preventing HNSCC metastasis mediated by SDF-1α.
CXCL12/stromal-derived factor-1α (SDF-1α)2 is a widely expressed chemotactic cytokine (chemokine) that selectively binds to the G protein-coupled receptor CXCR4. Chemokine gradients are able to induce a directed migration of cells that express the appropriate receptors. CXCR4, a 7-transmembrane domain-containing receptor, most notably functions as a coreceptor for human immunodeficiency virus entry into CD4+ T cells (1–2). SDF-1α was first identified in a bone marrow stromal cell line and was described to be involved in B cell maturation and the homing of hematopoietic progenitor cells to bone marrow stromal cell niches (3–6). Other studies have shown the involvement of SDF-1α/CXCR4 signaling in lymphocyte trafficking, hematopoiesis, vascularization, and fetal development (7).
More recently, CXCR4 has gained considerable attention for its role in tumor progression and metastasis as shown by numerous studies in solid and hematopoietic malignancies (8). Head and neck squamous cell carcinoma (HNSCC) is a very malignant tumor with a 5-year survival rate of only 50%. Significant improvements in the treatment of HNSCC have not been made in recent decades. The most important indicator of patient prognosis is the presence or absence of HNSCC lymph node metastasis. Studies have shown that oral squamous cell carcinoma has increased expression of CXCR4 and that expression levels are significantly correlated with lymph node metastasis, recurrence, and an overall poor prognosis (9–12). Further, CXCR4 expression has been found to be higher in metastatic HNSCC tissue compared with non-metastatic and normal tissue (13). SDF-1α signaling has also been shown to induce epithelial to mesenchymal transition of HNSCC, a process in which cells lose their epithelial characteristics and acquire a fibroblast-like phenotype in order to migrate, possible contributing to the dissemination of tumor cells (14). As studies have shown, the metastatic process of HNSCC may be accompanied by an increase in matrix metalloprotease secretion stimulated by SDF-1α (15). The tumor microenvironment also has a pivotal role in the progression of cancer as seen in carcinomas of the breast in which SDF-1α is secreted by tumor fibroblasts, contributing to the proliferation and survival of the tumor cells in a paracrine manner (16). SDF-1α can also recruit endothelial progenitor cells to promote tumor angiogenesis and can induce blood vessel instability and transendothelial migration as a mechanism to promote tumor metastasis (17). Because SDF-1α is constitutively expressed by stromal fibroblasts of specific organs, such as the liver, lungs, lymph nodes, and bone, neoplastic cells expressing CXCR4 may be able to home into these tissues to establish distant metastases; however, the mechanisms by which SDF-1α exerts its metastatic effect are largely unknown.
SDF-1α is able to activate a wide variety of distinct signaling pathways, including PI3K/Akt and p42/44 MAPK, but not stress-induced kinases such as p38 kinase and c-Jun amino-terminal kinase (18–19). One study also shows SDF-1α can indirectly activate NF-κB signaling through a MAPK-dependent increase in TNFα production (20). The NF-κB family of transcription factors includes RelA (p65), RelB, c-Rel, p50/p105, and p52/p100. These proteins play a crucial role in a variety of physiological and pathological events, including inflammation and immune responses, apoptosis, proliferation, and tumorigenesis (21). In the canonical pathway, NF-κB proteins are bound to inhibitory molecules (IκBs) and are sequestered in the cytoplasm in an inactive state. When cells are stimulated by appropriate factors, the IκB kinase (IKK) complex, containing catalytically active IKKα and IKKβ and a regulatory scaffold protein IKKγ/NEMO, phosphorylates IκB, leading to its ubiquitination and proteasomal destruction. NF-κB is subsequently released from inhibition to enter the nucleus and can either repress or activate gene transcription.
In this report, we hypothesize that NF-κB may play an important role in SDF-1α-induced invasion of HNSCC. We found that SDF-1α directly activated NF-κB by inducing IKK activity in HNSCC cells. We demonstrated that the mechanism of SDF-1α-induced NF-κB activation was both PI3K/Akt- and ERK-independent, as inhibition of these signaling pathways did not affect SDF-1α-mediated phosphorylation and degradation of IκBα. IKK activation was dependent on the CXCR4 receptor. In addition, contrary to recent studies, we found that TNFα neutralization did not have an effect on SDF-1α-induced IκBα phosphorylation. Furthermore, inhibition of IKKβ significantly inhibited HNSCC cell invasion induced by SDF-1α. These results suggest that targeting components of the NF-κB signaling pathway may be beneficial in preventing the progression and metastasis of HNSCC.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents—TB2-T1 and TCA8113 oral squamous cell carcinoma cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin-streptomycin. Normal human epidermal keratinocytes were purchased from Lonza and maintained in KGM-2 medium supplemented with insulin, hydrocortisone, transferrin, epinephrine, bovine pituitary extract, epidermal growth factor, and gentamicin. HOK16B human papillomavirus-immortalized keratinocytes were a kind gift from Drs. K. I.-Hyuk Shin and No-Hee Park and maintained in KGM medium (Lonza). Antibodies were obtained from the following companies: phospho-IκBα (Ser-32/36), phospho-Akt (Ser-473), Cell Signaling; phospho-ERK, ERK, Akt, and IκBα, Santa Cruz Biotechnology. IKK2-VI, LY294002, U0126, and AMD3100 inhibitors were purchased from Calbiochem. Recombinant human SDF-1α and anti-human TNFα-neutralizing antibody were purchased from R&D Systems.
Western Blotting—Cells were grown to 70% confluence in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Cells were rinsed two times with sterile phosphate-buffered saline, serum-starved overnight, and stimulated with 50 ng/ml SDF-1α for the indicated times. Normal human epidermal keratinocytes and HOK16B cells were growth factor-starved overnight and stimulated as described above. After treatment, cells were rinsed twice with cold phosphate-buffered saline, scraped, and pelleted. Cell pellets were lysed using radioimmunoprecipitation assay buffer containing protease inhibitor mixture and phenylmethylsulfonyl fluoride. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad) using a semidry transfer apparatus. Membranes were blocked for 1 h in 5% nonfat milk and incubated with primary antibody overnight at 4 °C. Membranes were washed in Tris-buffered saline Tween and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Proteins were detected using the enhanced chemiluminescence system (Pierce).
Electrophoretic Mobility Shift Assay—Cells were treated for the indicated times, and 10 μg of nuclear extract was incubated with 2 μg of poly(dI-dC) (Amersham Biosciences), DNA binding buffer (250 mm NaCl, 50 mm Tris, 50% glycerol, 5 mm dithiothreitol, 2.5 mm EDTA), and [γ-32P]CTP-labeled NF-κB consensus sequence. Extracts were electrophoresed on a 5% polyacrylamide gel and subjected to autoradiography.
TNFα Neutralization Study—Cells were serum-starved overnight, and the next day 10 μg/ml of control mouse IgG or monoclonal TNFα-blocking antibody was added directly to the medium along with either 10 ng/ml TNFα for 30 min or 50 ng/ml SDF-1α for the times indicated. Cells were lysed using radioimmune precipitation buffer, and Western blotting was performed as described.
Invasion Assay—Invasion assay was performed according to the manufacturer's protocol (BD Biosciences). Briefly, cells were seeded in serum-free medium with or without IKKβVI or AMD3100 inhibitors on growth factor-reduced Matrigel-coated membranes containing 8-μm pores. The lower chamber contained Dulbecco's modified Eagle's medium with 0.1% fetal bovine serum with or without SDF-1α. After 48 h, cells on the top of the membrane that had not migrated were gently removed with a cotton swab and cells that had migrated through the membrane were stained with Protocol™ Hema3 kit and counted under the microscope.
RESULTS
SDF-1α Induces Phosphorylation and Degradation of IκBα and Activates Akt and ERK1/2 in HNSCC—After appropriate stimulation, IκB inhibitory proteins were phosphorylated on specific serine residues, resulting in proteolysis and release of NF-κB to modulate gene transcription in the nucleus. To determine whether SDF-1α activates NF-κB, we first asked whether IκBα was phosphorylated and degraded in two human HNSCC cell lines. TB2-T1 and TCA8113 cells were treated with SDF-1α and analyzed for phosphorylation and degradation of IκBα by Western blotting (Fig. 1A). Both cell lines showed inducible phosphorylation of IκBα with slight activation around 5 min and a robust response at 30 and 60 min. This was correlated with an SDF-1α-induced degradation of IκBα at 30 and 60 min. Cell lysates were also analyzed for Akt and ERK activation by Western blotting using phospho-specific antibodies. Akt phosphorylation was induced within 5 min by SDF-1α in both cell lines and remained active up until 60 min with SDF-1α stimulation, whereas total Akt levels were unchanged. ERK activity was inducible in TB2-T1 cells, but we found that SDF-1α did not affect ERK activity in TCA8113 cells as it was constitutively activated. This result was not surprising to us as MAPK signaling has been shown to be chronically activated in HNSCC (22–23).
FIGURE 1.
SDF-1α activates IKK, Akt, and ERK in HNSCC. A, IKK is activated by SDF-1α in HNSCC. TB2-T1 and TCA8113 cells were serum-starved and treated with 50 ng/ml SDF-1α for various times. Cell lysates (50 μg) of treated or untreated samples were resolved on a 10% SDS-PAGE and subjected to immunoblotting using anti-phospho IκBα (Ser-32/36), anti-IκBα, anti-Akt (Ser-473), anti-Akt, anti-phospho-ERK1/2, anti-ERK, and anti-α-tubulin (loading control) antibodies. Proteins were visualized by enhanced chemoluminescence. B, SDF-1α does not activate IKK in primary or immortalized keratinocytes. Normal human epidermal keratinocytes and human papillomavirus-immortalized keratinocytes were growth factor-starved overnight and then stimulated with 50 ng/ml SDF-1α. Whole cell extracts were resolved on a 10% SDS-PAGE and blotted using antibodies against phospho-IκBα, IκBα, and α-tubulin.
Normal human epidermal keratinocytes and HOK16B human papillomavirus-immortalized keratinocytes were also analyzed for SDF-1α-induced IκBα phosphorylation and degradation (Fig. 1B). We found that there was no SDF-1α-mediated phosphorylation and degradation of IκBα in primary keratinocytes. Normal human epidermal keratinocytes showed an overall lower expression level of IκBα protein compared with HOK16B and TB2-T1 cells. HOK16B cells showed slight phosphorylation of IκBα compared with TB2-T1 cells; however, this was independent of SDF-1α stimulation. IκBα phosphorylation was visible in untreated cells and was not further induced in response to SDF-1α, suggesting that NF-κB may be constitutively activated in human papillomavirus-immortalized keratinocytes.
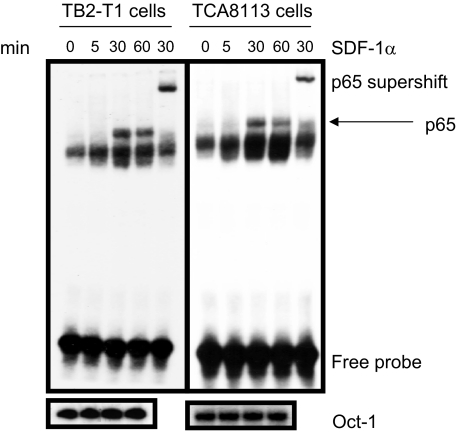
SDF-1α Stimulation Causes p65 Translocation to the Nucleus in HNSCC—The NF-κB transcription factors are released from inhibition after IκB proteins are phosphorylated by the IKK complex and degraded by the ubiquitin-proteasome machinery. Thus, we sought to investigate whether NF-κB was translocated to the nucleus after SDF-1α stimulation of HNSCC. Nuclear proteins were purified from HNSCC cells after treatment with SDF-1α for various times. We performed electrophoretic mobility shift assay to visualize NF-κB binding to a κB consensus sequence often found in promoter regions of target genes. Electrophoretic mobility shift assay revealed that an SDF-1α-stimulated induction of NF-κB nuclear translocation (Fig. 2) began at 30 min and lasted through 60 min in TB2-T1 and TCA8113 cells, which corresponded to the phosphorylation and degradation of IκBα.
FIGURE 2.
SDF-1α induces nuclear translocation of NF-κB in HNSCC. TB2-T1 and TCA8113 cells were serum-starved and treated with 50 ng/ml SDF-1α for various times. Nuclear proteins (10 μg) from treated and untreated samples were isolated and incubated with [γ-32P]CTP-labeled NF-κB probe and DNA binding buffer for 30 min at room temperature. Electrophoretic mobility shift assay was performed, and samples were separated on a 5% polyacrylamide gel. Protein-DNA complexes were visualized by autoradiography. Supershift lane represents nuclear extracts of cells treated with SDF-1α for 30 min and then incubated with p65 antibody for 30 min at room temperature, followed by addition of NF-κB probe. Oct-1 nuclear protein served as loading control using 32P-labeled Oct-1 DNA probe.
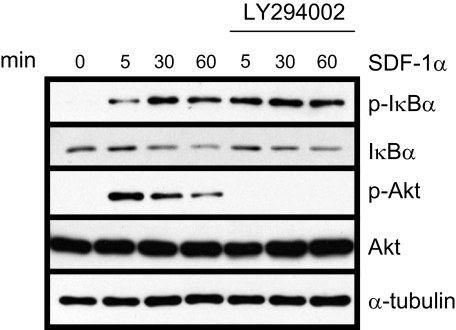
Inhibition of Both PI3K/Akt and MAPK Pathways Has No Significant Effect on IκBα Phosphorylation and Degradation—The PI3K/Akt signaling pathway has been shown to activate NF-κB. Because SDF-1α can activate PI3K/Akt signaling, we asked whether this pathway might be responsible for activating NF-κB in HNSCC cells. TB2-T1 cells were pretreated with LY294002 (PI3K inhibitor) and then stimulated with SDF-1α. Western blotting was performed to examine IκBα phosphorylation and degradation (Fig. 3). SDF-1α-mediated phosphorylation of Akt was effectively ablated by inhibition of PI3K using 10 μm LY294002. In contrast, SDF-1α-induced IκBα phosphorylation and degradation in TB2-T1 cells was not blocked by treatment with LY294002. This suggests that the PI3K/Akt signaling pathway does not lie upstream of NF-κB and is not responsible for SDF-1α-induced NF-κB activity in HNSCC cells.
FIGURE 3.
SDF-1α-induced IKK is PI3K/Akt-independent in HNSCC. TB2-T1 cells were serum-starved overnight and then treated with 10 μm PI3K inhibitor LY294002 for 60 min. Cells were then stimulated with 50 ng/ml SDF-1α for various times. Lysates were prepared, and 50 μg of protein was separated on 10% SDS-PAGE and subjected to immunoblotting with antibodies for phospho-IκBα (Ser-32/36), IκBα, phospho-Akt (Ser-473), Akt, and α-tubulin (loading control).
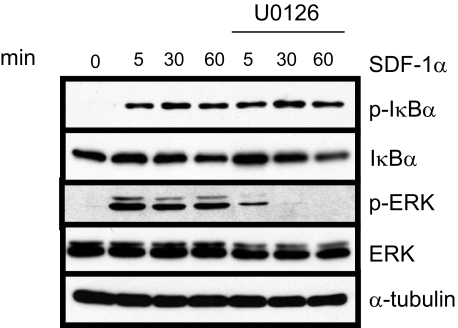
Numerous reports have shown that CXCR4 can activate the MAPK signaling pathway, and other studies have shown NF-κB activation by MAPK (24–26). One study showed that SDF-1α-induced NF-κB activity in prostate cancer cells can be blocked with treatment of MAPK inhibitors (27). Therefore, next we examined whether this might be true in HNSCC as well. We pretreated TB2-T1 cells with 10 μm MAPK inhibitor U0126 for 60 min and then stimulated with SDF-1α for the indicated times (Fig. 4). We were able to block SDF-1α-mediated ERK1/2 activation using the MAPK inhibitor, but there was no significant change in SDF-1α-induced IκBα phosphorylation and degradation, indicating that the ERK1/2 MAPK cascade is probably not responsible for activating NF-κB in response to SDF-1α.
FIGURE 4.
SDF-1α-induced IKK is MAPK-independent in HNSCC. TB2-T1 cells were serum-starved overnight and then treated with 10 μm MAPK inhibitor U0126 for 60 min. Cells were then stimulated with 50 ng/ml SDF-1α for various times. Lysates were prepared, and 50 μg of protein was separated on 10% SDS-PAGE and subjected to immunoblotting with antibodies for phospho-IκBα (Ser-32/36), IκBα, phospho-ERK 1/2, ERK, and α-tubulin (loading control).
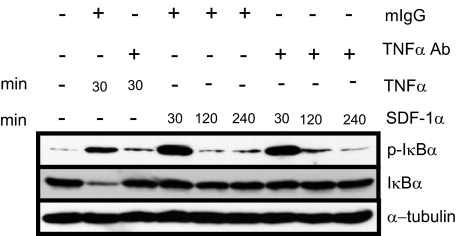
TNFα Does Not Mediate SDF-1α-induced NF-κB Activation—Han et al. (20) have reported that NF-κB activation by SDF-1α is indirectly mediated by TNFα such that TNFα protein synthesis and secretion is up-regulated as a result of SDF-1α activation of MAPK in primary astrocytes. In turn, the newly synthesized and secreted TNFα activates NF-κB signaling in an autocrine manner. We sought to test whether TNFα-neutralizing antibodies could abolish TNFα-induced NF-κB activation in HNSCC. Western blot analysis showed that TNFα strongly induced IκBα phosphorylation and that TNFα neutralization was able to effectively inhibit TNFα-mediated IκBα phosphorylation (Fig. 5). However, TNFα neutralization did not affect SDF-1α-induced IκBα phosphorylation. These results suggest that SDF-1α-induced NF-κB activity in HNSCC cells is not indirectly mediated by TNFα synthesis and secretion.
FIGURE 5.
SDF-1α-induced IKK is independent of TNFα-mediated signaling in HNSCC. TB2-T1 cells were serum-starved overnight and treated with 10 ng/ml TNFα for 30 min (lanes 2 and 3) or 50 ng/ml SDF-1α for the indicated times. Control mouse IgG or TNFα-neutralizing antibody was also added directly to the medium along with stimulatory factors. Total lysates were harvested, and 50 μg of protein was separated on 10% SDS-PAGE and subjected to immunoblotting using phospho-IκBα, IκBα, and α-tubulin (loading control) antibodies.
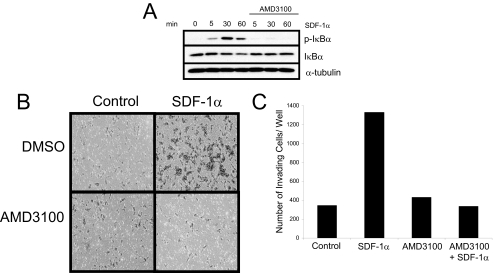
SDF-1α-mediated Activation of NF-κB and HNSCC Invasion Is CXCR4-dependent—Recent reports have shown that in addition to binding to CXCR4, SDF-1α can bind to another chemokine receptor, CXCR7 (28). Thus, we used a CXCR4 antagonist, AMD3100, to determine whether the activation of NF-κB is due to SDF-1α binding to CXCR4 or if there could possibly be another receptor that could be signaling to NF-κB. TB2-T1 cells were pretreated with AMD3100 and then stimulated with SDF-1α for the indicated times (Fig. 6A). Western blot analysis of the protein lysates showed that AMD3100 was able to markedly reduce SDF-1α-mediated IκBα phosphorylation and degradation, suggesting that activation of NF-κB signaling occurs through the CXCR4 receptor. We also investigated whether SDF-1α-mediated invasion of HNSCC occurred through the CXCR4 receptor, using a Matrigel chamber assay that mimics the invasiveness and metastasis of HNSCC in vivo. TB2-T1 cells treated with 10 μm AMD3100 showed a significant reduction in SDF-1α-mediated invasion compared with cells treated with SDF-1α only (Fig. 6, B and C), suggesting that SDF-1α-mediated HNSCC invasion is CXCR4-dependent.
FIGURE 6.
SDF-1α-induced IKK activation and HNSCC invasion are CXCR4-dependent. A, CXCR4 inhibitor blocks IκBα phosphorylation and degradation. TB2-T1 cells were serum-starved overnight and pretreated with 10 μm CXCR4 antagonist AMD3100 for 1 h. Cells were stimulated with 50 ng/ml SDF-1α for the indicated times. Whole cell lysates were prepared, and 50 μg of protein were resolved on a 10% SDS-PAGE and subjected to immunoblotting using phospho-IκBα, IκBα, and α-tubulin (loading control) antibodies. B and C, CXCR4 inhibitor blocks SDF-1α-induced HNSCC invasion. Medium containing 0.1% fetal bovine serum with or without 50 ng/ml SDF-1α was added to the lower well of the invasion chamber. TB2-T1 cells were seeded in the upper chamber with serum-free medium with or without 10 μm AMD3100. After 48 h, cells that had migrated were stained with hematoxylin and eosin. Cells that invaded through the Matrigel-coated inserts were counted. Invasion assay was repeated three times.
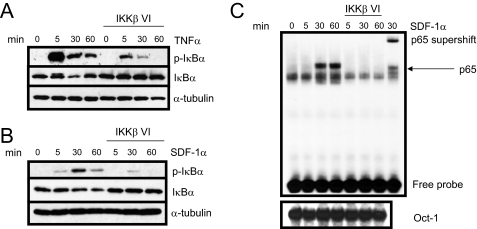
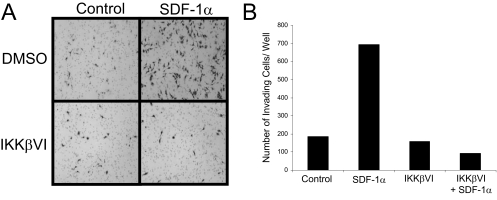
Inhibition of IKKβ Blocks Invasion of HNSCC—We and others have shown that inhibition of the NF-κB pathway has proven to be effective in preventing tumor cell proliferation, migration, and invasion in vitro as well as inhibiting tumor growth and metastasis in vivo (29–30). To test whether NF-κB plays a role in SDF-1α-induced HNSCC cell invasion, we utilized a specific inhibitor of IKKβ to block NF-κB activation. As a control, TNFα was used to test the efficacy of the inhibitor in TB2-T1 cells. Pretreatment of TB2-T1 cells with 1 μm IKKβ inhibitor followed by TNFα or SDF-1α stimulation significantly inhibited the phosphorylation and degradation of IκBα as shown by Western blot analysis (Fig. 7, A and B). Furthermore, electrophoretic mobility shift assay analysis showed that SDF-1α-mediated nuclear translocation of p65 was dramatically suppressed in TB2-T1 cells treated with the IKKβ inhibitor (Fig. 7C). To determine whether the IKKβ inhibitor blocks HNSCC cell invasion, next we performed a Matrigel chamber assay. In response to SDF-1α stimulation, a significant amount of TB2-T1 cells were able to invade through Matrigel after 48 h compared with untreated cells. With the addition of 1 μm IKKβ inhibitor directly to the upper chamber containing the cells, we observed a dramatic decline in the invasion of TB2-T1 cells in response to SDF-1α (Fig. 8). We found that the use of the IKKβ inhibitor did not affect the proliferation of TB2-T1 cells, eliminating the possibility of a reduction in invasion due to a block in proliferation (supplemental Fig. S1). These data suggest that SDF-1α promotes HNSCC invasion through activating NF-κB.
FIGURE 7.
IKKβ inhibitor blocks SDF-1α-induced NF-κB activation in HNSCC. A and B, IKKβ inhibitor reduces SDF-1α- and TNFα-induced IκBα phosphorylation and degradation. TB2-T1 cells were serum-starved overnight and pretreated with 1 μm IKKβ inhibitor for 30 min. Cells were stimulated with either 10 ng/ml TNFα or 50 ng/ml SDF-1α for the indicated times. Total protein was harvested, and lysates (50 μg) were resolved on a 10% SDS-PAGE and analyzed by Western blotting using antibodies against phospho-IκBα (Ser-32/36), IκBα, and α-tubulin (loading control). C, IKKβ inhibitor prevents nuclear translocation of NF-κB. TB2-T1 cells were treated as above, and nuclear extracts were purified and incubated with radiolabeled NF-κB DNA probe and DNA binding buffer. Electrophoretic mobility shift assay was performed using 10 μg of nuclear extract. Protein-DNA complexes were visualized by autoradiography. Supershift lane represents nuclear extract of cells treated with 50 ng/ml SDF-1α for 30 min with NF-κB DNA probe and p65 antibody. Oct-1 served as loading control.
FIGURE 8.
Inhibition of NF-κB blocks SDF-1α-induced HNSCC invasion. A, invasive HNSCC cell staining by hematoxylin and eosin. Medium containing 0.1% fetal bovine serum with or without 50 ng/ml SDF-1α was added to the lower well of the invasion chamber. TB2-T1 cells were seeded in the upper chamber with serum-free medium with or without 1 μm IKKβ inhibitor. After 48 h, cells that had invaded to the lower chamber, toward SDF-1α, were stained. B, SDF-1α induces TB2-T1 cell invasion and IKKβ inhibitor blocks SDF-1α-induced invasion. Cells that invaded through the Matrigel-coated inserts were counted. Invasion assay was repeated three times.
DISCUSSION
The chemokine receptor CXCR4 and its ligand SDF-1α have gained considerable attention because of their roles in tumor progression and metastasis. CXCR4 is overexpressed in carcinomas of the breast, ovaries, cervix, prostate, brain, lung, thyroid, and colon (30). Signaling mediated by the SDF-1α/CXCR4 axis promotes tumorigenesis by enhancing cancer cell growth and survival, migration away from the primary tumor, and establishment of metastases in distant organs (31–34). Given the high profile role of CXCR4 signaling in the development and progression of a variety of cancers, it is of interest and importance to produce therapies targeting this molecule or its downstream signaling partners to combat these fatal diseases. Indeed, CXCR4 neutralization has shown promise in preventing tumor progression and metastasis in vitro and in vivo (32, 35–41).
NF-κB has been shown to regulate the gene expression of many metastasis-promoting factors, and NF-κB is also constitutively activated in a variety of cancers, including HNSCC (42–46). Currently there are no studies showing that SDF-1α can activate NF-κB signaling in HNSCC, and a clear role for NF-κB in SDF-1α-mediated invasion has not been fully described. In this report we identified NF-κB as a downstream component of CXCR4 signaling and suggest that targeting this pathway may be useful in blocking SDF-1α-mediated HNSCC tumor invasion and metastasis. We have shown that SDF-1α can induce the phosphorylation and degradation of IκBα and cause nuclear translocation of the NF-κB p65 subunit in HNSCC. Importantly, SDF-1α-mediated activation of NF-κB signaling was absent in both primary and immortalized keratinocytes, suggesting that CXCR4 and/or its downstream signaling components may be abnormally regulated in HNSCC. Blocking SDF-1α-induced NF-κB signaling using an IKKβ inhibitor significantly inhibited the phosphorylation and degradation of IκBα, p65 nuclear translocation, and, importantly, interfered with the invasion of HNSCC. Although we showed that NF-κB activity was inducible in vitro in our cell lines, the tumor microenvironment may promote constitutive activation of NF-κB and an increase in tumor invasion mediated by SDF-1α secretion in vivo.
Interestingly, chemokines and chemokine receptors are an important class of NF-κB target genes. Enhancing the expression of these molecules through NF-κB activity may contribute to the metastatic spread of cancer. Several reports suggest that there are NF-κB binding elements in the CXCR4 promoter region and that CXCR4 receptor expression can be enhanced by NF-κB signaling (27, 47). A study in prostate cancer has shown that SDF-1α can enhance tumor cell adhesion to human umbilical vein endothelial cells and induce transendothelial migration. SDF-1α-mediated activation of NF-κB can up-regulate CXCR4 expression, and the use of a dominant negative IκBα or anti-CXCR4 antibodies can suppress these events (27). A study in breast cancer cells has also shown that constitutively active NF-κB can up-regulate CXCR4 expression, thus promoting tumor cell migration and metastasis (47). These studies and our results indicate that SDF-1α-CXCR4-NF-κB signaling may be cyclical and can be amplified to further contribute to tumor progression because SDF-1α can activate NF-κB and NF-κB can increase CXCR4 expression.
CXCR4, which is a pertussis toxin-sensitive G protein-coupled receptor, can activate a number of signaling pathways that are both G protein-dependent and -independent. Because SDF-1α is able to activate a variety of signaling pathways that can influence NF-κB activity, we sought to determine which, if any, are involved in HNSCC. Most of the current work on SDF-1α-dependent invasion and metastasis focuses on the roles of PI3K/Akt and MAPK signaling pathways. Studies in prostate cancer have shown that blocking the MAPK pathway can inhibit SDF-1α-mediated NF-κB signaling; however, we found that blocking MAPK signaling had no significant effect on NFκB activity in HNSCC, suggesting a non-linear and separate pathway (27). Additional studies have shown that SDF-1α can induce NF-κB activity in primary astrocytes via an indirect mechanism activated by MAPK that involves de novo synthesis of TNFα (20). We tested this possibility in our system and found that TNFα had little to do with SDF-1α-induced NF-κB activity because TNFα neutralization did not change SDF-1α-mediated IκBα phosphorylation. It is also important to note that we observed IκBα phosphorylation as early as 5 min, allowing little time for de novo synthesis and secretion of TNFα. Furthermore, we were able to show that PI3K/Akt signaling does not affect NF-κB activity in HNSCC. Our results suggest there is a distinct and previously unknown pathway in HNSCC that may be involved in directly activating IKK/NF-κB signaling in response to SDF-1α. We are currently undertaking efforts to identify signaling molecules that may be involved. Importantly, we found that the inhibition of NF-κB blocks HNSCC invasion induced by SDF-1α, indicating that SDF-1α may activate NF-κB to promote HNSCC progression and metastasis in vivo. Targeting IKK/NF-κB signaling may help to develop a new strategy in preventing HNSCC metastasis.
Supplementary Material
Acknowledgments
We thank Drs. Ki-Hyuk Shin and No-Hee Park for providing immortalized keratinocytes.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: SDF-1α, stromal cell-derived factor-1α; HNSCC, head and neck squamous cell carcinoma; NF-κB, nuclear factor-κB; IκB, inhibitor of κB; IKK, IκB kinase; TNFα, tumor necrosis factor α; PI3K, phosphoinositide 3-kinase; ERK, extracellular-regulated kinase; MAPK, mitogen-activated protein kinase.
References
- 1.Nagasawa, T., Nakajima, T., Tachibana, K., Iizasa, H., Bleul, C. C., Yoshie, O., Matsushima, K., Yoshida, N., Springer, T. A., and Kishimoto, T. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 14726–14729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng, Y., Broder, C. C., Kennedy, P. E., and Berger, E. A. (1996) Science 272 872–877 [DOI] [PubMed] [Google Scholar]
- 3.Tashiro, K., Tada, H., Heilker, R., Shirozu, M., Nakano, T., and Honjo, T. (1993) Science 261 600–603 [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa, T., Kikutani, H., and Kishimito, T. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 2305–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagasawa, T., Hirota, S., Tachibana, K., Takakura, N., Nishikawa, S., Kitamura, Y., Yoshida, N., Kikutani, H., and Kishimoto, T. (1996) Nature 382 635–638 [DOI] [PubMed] [Google Scholar]
- 6.Murdoch, C. (2000) Immunol. Rev. 177 175–184 [DOI] [PubMed] [Google Scholar]
- 7.Rossi, D., and Zlotnik, A. (2000) Annu. Rev. Immunol. 18 217–242 [DOI] [PubMed] [Google Scholar]
- 8.Burger, J. A., and Kipps, T. J. (2006) Blood 107 1761–1767 [DOI] [PubMed] [Google Scholar]
- 9.Almofti, A., Uchida, D., Begum, N. M., Tomizuka, Y., Iga, H., Yoshida, H., and Sato, M. (2004) Int. J. Oncol. 25 65–71 [PubMed] [Google Scholar]
- 10.Ishikawa, T., Nakashiro, K., Hara, S., Klosek, S., Li, C., Shintani, S., and Hamakawa, H. (2006) Int. J. Oncol. 28 61–66 [PubMed] [Google Scholar]
- 11.Uchida, D., Begum, N. M., Almofti, A., Nakashiro, K., Kawamata, H., Tateishi, Y., Hamakawa, H., Yoshida, H., and Sato, M. (2003) Exp. Cell Res. 290 289–302 [DOI] [PubMed] [Google Scholar]
- 12.Uchida, D., Begum, N. M., Tomizuka, Y., Banda, T., Almofti, A., Yoshida, H., and Sato, M. (2004) Lab. Investig. 84 1538–1546 [DOI] [PubMed] [Google Scholar]
- 13.Delilbasi, C. B., Okura, M., and Kogo, M. (2004) Oral Oncol. 40 154–157 [DOI] [PubMed] [Google Scholar]
- 14.Onoue, T., Uchida, D., Begum, N. M., Tomizuka, Y., Yoshida, H., and Sato, M. (2006) Int. J. Oncol. 29 1133–1138 [PubMed] [Google Scholar]
- 15.Samara, G. J., Lawrence, D. M., Chiarelli, C. J., Valentino, M. D., Lyubsky, S., Zucker, S., and Vaday, G. G. (2004) Cancer Lett. 214 231–241 [DOI] [PubMed] [Google Scholar]
- 16.Orimo, A., Gupta, P. B., Sgroi, D. C., Arenzana-Seisdedos, F., Delaunay, T., Naeem, R., Carey, V. J., Richardson, A. L., and Weinberg, R. A. (2005) Cell 121 335–348 [DOI] [PubMed] [Google Scholar]
- 17.Lee, B. C., Lee, T. H., Avraham, S., and Avraham, H. K. (2004) Mol. Cancer Res. 2 327–338 [PubMed] [Google Scholar]
- 18.Ganju, R. K., Brubaker, S. A., Meyer, J., Dutt, P., Yang, Y., Qin, S., Newman, W., and Groopman, J. E. (1998) J. Biol. Chem. 273 23169–23175 [DOI] [PubMed] [Google Scholar]
- 19.Lee, Y., Gotoh, A., Kwon, H. J., You, M., Kohli, L., Mantel, C., Cooper, S., Hangoc, G., Miyazawa, K., Ohyashiki, K., and Broxmeyer, H. E. (2002) Blood 99 4307–4317 [DOI] [PubMed] [Google Scholar]
- 20.Han, Y., Huang, D., Pardo, C. A., and Ransohoff, R. M. (2001) J. Clin. Investig. 108 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karin, M., Cao, Y., Greten, F. R., and Li, Z. W. (2002) Nat. Rev. Cancer 2 301–310 [DOI] [PubMed] [Google Scholar]
- 22.Patel, V., Leethanakul, C., and Gutkind, J. S. (2001) Crit. Rev. Oral Biol. Med. 12 55–63 [DOI] [PubMed] [Google Scholar]
- 23.Mao, L., Hong, W. K., and Papadimitrakopoulou, V. A. (2004) Cancer Cell 5 311–316 [DOI] [PubMed] [Google Scholar]
- 24.Zhao, Q., and Lee, F. S. (1999) J. Biol. Chem. 274 8355–8358 [DOI] [PubMed] [Google Scholar]
- 25.Nakano, H., Shindo, M., Sakon, S., Nishinaka, S., Mihara, M., Yagita, H., and Okumura, K. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3537–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemoto, S., DiDonato, J. A., and Lin, A. (1998) Mol. Cell. Biol. 18 7336–7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kukreja, P., Abdel-Mageed, A. B., Mondal, D., Liu, K., and Agrawal, K. C. (2005) Cancer Res. 65 9891–9898 [DOI] [PubMed] [Google Scholar]
- 28.Balabanian, K., Lagane, B., Infantino, S., Chow, K. Y. C., Harriague, J., Moepps, B., Arenzana-Seisdedos, F., Thelen, M., and Bachelerie, F. (2005) J. Biol. Chem. 280 35760–35766 [DOI] [PubMed] [Google Scholar]
- 29.Duffey, D. C., Chen, Z., Dong, G., Ondrey, F. G., Wolf, J. S., Brown, K., Siebenlist, U., and Van Waes, C. (1999) Cancer Res. 59 3468–3474 [PubMed] [Google Scholar]
- 30.Park, B. K., Zhang, H., Zeng, Q., Dai, J., Keller, E. T., Giordano, T., Gu, K., Shah, V., Pei, L., Zarbo, R. J., McCauley, L., Shi, S., Chen, S., and Wang, C. Y. (2007) Nat. Med. 13 62–69 [DOI] [PubMed] [Google Scholar]
- 31.Balkwill, F. (2004) Semin. Cancer Biol. 14 171–179 [DOI] [PubMed] [Google Scholar]
- 32.Müller, A., Homey, B., Soto, H., Ge, N., Catron, D., Buchanan, M. E., McClanahan, T., Murphy, E., Yuan, W., Wagner, S. N., Barrera, J. L., Mohar, A., Verástegui, E., and Zlotnik, A. (2001) Nature 410 50–56 [DOI] [PubMed] [Google Scholar]
- 33.Smith, M. C., Luker, K. E., Garbow, J. R., Prior, J. L., Jackson, E., Piwnica-Worms, D., and Luker, G. D. (2004) Cancer Res. 64 8604–8612 [DOI] [PubMed] [Google Scholar]
- 34.Phillips, R. J., Burdick, M. D., Lutz, M., Belperio, J. A., Keane, M. P., and Strieter, R. M. (2003) Am. J. Respir. Crit. Care Med. 167 1676–1686 [DOI] [PubMed] [Google Scholar]
- 35.Zeelenberg, I. S., Ruuls-Van Stalle, L., and Roos, E. (2001) J. Clin. Investig. 108 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeelenberg, I. S., Ruuls-Van Stalle, L., and Roos, E. (2003) Cancer Res. 63 3833–3839 [PubMed] [Google Scholar]
- 37.Bertolini, F., Dell'Agnola, C., Mancuso, P., Rabascio, C., Burlini, A., Monestiroli, S., Gobbi, A., Pruneri, G., and Martinelli, G. (2002) Cancer Res. 62 3106–3112 [PubMed] [Google Scholar]
- 38.Chen, Y., Stamatoyannopoulos, G., and Song, C. Z. (2003) Cancer Res. 63 4801–4804 [PubMed] [Google Scholar]
- 39.Rubin, J. B., Kung, A. L., Klein, R. S., Chan, J. A., Sun, Y., Schmidt, K., Kieran, M. W., Luster, A. D., and Segal, R. A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 13513–13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katayama, A., Ogino, T., Bandoh, N., Nonaka, S., and Harabuchi, Y. (2005) Clin. Cancer Res. 11 2937–2946 [DOI] [PubMed] [Google Scholar]
- 41.Scotton, C. J., Wilson, J. L., Scott, K., Stamp, G., Wilbanks, G. D., Fricker, S., Bridger, G., and Balkwill, F. R. (2002) Cancer Res. 62 5930–5938 [PubMed] [Google Scholar]
- 42.Andela, V. B., Schwarz, E. M., Puzas, J. E., O'Keefe, R. J., and Rosier, R. N. (2000) Cancer Res. 60 6557–6562 [PubMed] [Google Scholar]
- 43.Duffey, D. C., Crowl-Bancroft, C. V., Chen, Z., Ondrey, F. G., Nejad-Sattari, M., Dong, G., and Van Waes, C. (2000) Br. J. Cancer 83 1367–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ondrey, F. G., Dong, G., Sunwoo, J., Chen, Z., Wolf, J. S., Crowl-Bancroft, C. V., Mukaida, N., and Van Waes, C. (1999) Mol. Carcinog. 26 119–129 [DOI] [PubMed] [Google Scholar]
- 45.Dong, G., Chen, Z., Kato, T., and Van Waes, C. (1999) Cancer Res. 59 3495–3504 [PubMed] [Google Scholar]
- 46.Basseres, D. S., and Baldwin, A. S. (2006) Oncogene 25 6817–6830 [DOI] [PubMed] [Google Scholar]
- 47.Helbig, G., Christopherson, K. W., Bhat-Nakshatri, P., Kumar, S., Kishimoto, H., Miller, K. D., Broxmeyer, H. E., and Nakshatri, H. (2003) J. Biol. Chem. 278 21631–21638 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.