Abstract
The ω-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) possesses potent anti-inflammatory properties and has shown therapeutic benefit in numerous inflammatory diseases. However, the molecular mechanisms of these anti-inflammatory properties are poorly understood. DHA is highly susceptible to peroxidation, which yields an array of potentially bioactive lipid species. One class of compounds are cyclopentenone neuroprostanes (A4/J4-NPs), which are highly reactive and similar in structure to anti-inflammatory cyclopentenone prostaglandins. Here we show that a synthetic A4/J4-NP, 14-A4-NP (A4-NP), potently suppresses lipopolysaccharideinduced expression of inducible nitric-oxide synthase and cyclooxygenase-2 in macrophages. Furthermore, A4-NP blocks lipopolysaccharide-induced NF-κB activation via inhibition of Iκ kinase-mediated phosphorylation of IκBα. Mutation on Iκ kinase β cysteine 179 markedly diminishes the effect of A4-NP, suggesting that A4-NP acts via thiol modification at this residue. Accordingly, the effects of A4-NP are independent of peroxisome proliferator-activated receptor-γ and are dependent on an intact reactive cyclopentenone ring. Interestingly, free radical-mediated oxidation of DHA greatly enhances its anti-inflammatory potency, an effect that closely parallels the formation of A4/J4-NPs. Furthermore, chemical reduction or conjugation to glutathione, both of which eliminate the bioactivity of A4-NP, also abrogate the anti-inflammatory effects of oxidized DHA. Thus, we have demonstrated that A4/J4-NPs, formed via the oxidation of DHA, are potent inhibitors of NF-κB signaling and may contribute to the anti-inflammatory actions of DHA. These findings have implications for understanding the anti-inflammatory properties of ω-3 fatty acids, and elucidate novel interactions between lipid peroxidation products and inflammation.
Considerable evidence suggests that dietary consumption of ω-3 fatty acids reduces inflammation in vivo (1). Docosahexaenoic acid (DHA)2 and eicosapentaenoic acid (EPA), the two most abundant n-3 fatty acids in marine fish oils, have been shown to exert potent anti-inflammatory effects both in vitro and in vivo (2). Supplementation of humans with DHA and EPA has emerged as a therapeutic option for the treatment of a wide variety of inflammatory diseases, with DHA showing greater potency in some studies (3–5). However, the molecular mechanisms by which DHA exerts its effects are still poorly understood. DHA can inhibit the nuclear factor-κB (NF-κB) pathway in a variety of cell types, and this action contributes to its anti-inflammatory effect (6, 7). NF-κB is considered a primary mediator of the inflammatory response and regulates the transcription of a vast array of pro-inflammatory proteins, including inducible nitric-oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in response to diverse stimuli such as lipopolysaccharide (LPS) (8). However, the mechanism by which DHA inhibits NF-κB is unknown. Recent work has suggested that metabolism of DHA yields bioactive compounds that mediate its effects. Serhan and co-workers (9, 10) have described novel anti-inflammatory hydroxylated DHA metabolites (termed protectins and D-series resolvins) that are derived from the enzymatic lipoxygenase-mediated oxidation of DHA. These findings have led to considerable interest in determining other bioactive metabolites of DHA that may mediate the anti-inflammatory effects of this fatty acid.
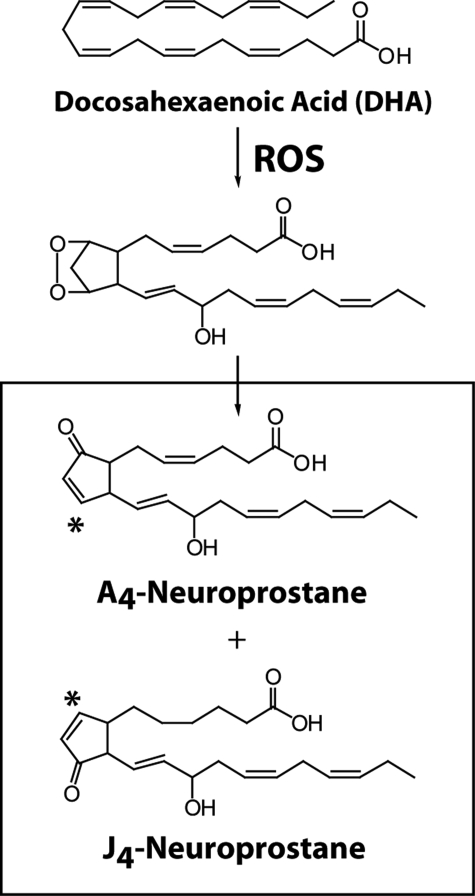
DHA is highly susceptible to free radical-mediated peroxidation, which gives rise to a wide array of unique lipid peroxidation products (11). Indeed, autooxidation of DHA occurs so readily that oxidation products are commonly found in DHA even after minimal handling (12). We have previously determined that oxidation of DHA yields a family of prostaglandin (PG)-like molecules that we have termed “neuroprostanes” (NPs), as these molecules are formed abundantly in DHA-rich tissues (such as the brain) in vivo (13). One class of endogenous NPs, the A4/J4-NPs, have been described, which are analogous in structure to the anti-inflammatory cyclopentenone PGs PGA2 and PGJ2 (Fig. 1). These molecules contain an electrophilic α,β-unsaturated carbonyl moiety that readily forms adducts with cellular thiols via Michael addition (14). Arachidonic acid-derived cyclopentenone eicosanoids potently suppress NF-κB signaling through covalent adduction (15, 16), suggesting that A4/J4-NPs might also possess these anti-inflammatory properties. We have previously demonstrated the formation of A4/J4-NPs in vivo in animals and have found that they are highly abundant in DHA-rich tissues (14). However, the bioactivity of A4/J4-NPs has never been examined. We have recently obtained one A-ring NP that we have shown is formed in vivo, 14-A4-NP (A4-NP), via chemical synthesis. This has allowed us the opportunity to examine, for the first time, the biological activity of A4/J4-NPs. Because of their structural similarities to anti-inflammatory cyclopentenone PGs and IsoPs, we hypothesize that A4/J4-NPs exert anti-inflammatory actions and may mediate some of the bioactivity of DHA.
FIGURE 1.
Formation of A4/J4-NPs via free radical-mediated oxidation of DHA. The A4/J4-NP regioisomers shown in the figure are 14-series compounds. For simplicity, stereochemistry is not shown. * denotes the electrophilic carbon. ROS, reactive oxygen species.
EXPERIMENTAL PROCEDURES
Reagents—A4-NP as a racemic mixture (Fig. 1) was obtained by total synthesis (17). A4-NP was stored in ethyl acetate at –80 °C until immediately before use at which time it was dried under nitrogen and resuspended in ethanol. A4-NP was added to culture medium immediately before its addition to cells, and because A4-NP rapidly reacts with albumin, serum-free medium was used for all experiments involving A4-NP, DHA, and oxDHA. A4-NP was stable in culture media for >4 h. DHA was obtained from NuChek Prep (Elysian, MN) and was stored at –80 °C under argon. oxDHA was prepared by adding 5 mm 2,2′-azobis-(2-amidinopropane) hydrochloride (AAPH), a free radical initiator, and incubating at 37 °C for various times. IKKβ (C179A) mutant expression vector was a gift of Dr. M. Karin (University of California, San Diego). Lipopolysaccharide (LPS) (from Salmonella Minnesota Re 595), was from Sigma, and tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) were obtained from R & D Systems (Minneapolis, MN). IκBα and p65 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), and iNOS, phospho-IκBα, and extracellular signal-related kinase (ERK) antibodies were from Cell Signaling Technologies (Danvers, MA). COX-2 polyclonal antibody, GW9662, and T00907 were obtained from Cayman Chemical Co. (Ann Arbor, MI). All cell culture media and supplies were from Invitrogen unless otherwise noted.
Cell Culture—RAW267.4 murine macrophage cells and HEK293 cells were obtained from ATCC (Manassas, VA). NF-κB reporter macrophages were obtained from the bone marrow of transgenic mice expressing a reporter plasmid containing the human immunodeficiency virus-long terminal repeat 36-bp enhancer (containing a total of eight NF-κB-binding sites) upstream of the herpes simplex virus minimal thymidine kinase promoter driving expression of Photinus luciferase (18). All cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin and plated on 24-well plates or 100-mm tissue culture dishes 24 h before experimentation.
Immunofluorescence Microscopy—For immunofluorescence staining, RAW cells were grown on glass coverslips. Following exposure to A4-NP or vehicle, cultures were fixed in 10% formaldehyde for 20 min, rinsed with PBS, permeabilized with 0.1% Triton X-100, and blocked for 1 h with 8% bovine serum albumin diluted in PBS. Coverslips were then incubated overnight at 4 °C in rabbit anti-p65 (1:100) primary antibody in 1% bovine serum albumin. Cells were washed in PBS for a total of 20 min and incubated in Cy-2-labeled secondary antibodies for 1 h. Cells were then washed again and stained with 1.4 μm 4′,6-diamidino-2-phenylindole for 10 min followed by further washes. Coverslips were mounted on microscope slides, and fluorescence was visualized with a Zeiss Axioplan microscope.
NF-κB Reporter Assay—NF-κB reporter macrophages were employed for NF-κB reporter assays. Following treatment, cells were scraped at select time points in Lysis Buffer (Promega, Madison, WI). The cell debris was pelleted by centrifugation at 13,000 rpm for 5 min, and 20 μl of the sample was used to perform a standard luciferase assay using a luminometer.
IKK Transfection—HEK293 cells were transfected with NF-κB-luc reporter plus empty vector, WT, or mutant (C179A) IKKβ expression vectors using the Geneporter 2 system (Genlantis, San Diego). 48 h after transfection, cells were treated as described above, then harvested, and subjected to luciferase reporter assay.
Western Blotting—Total protein extract was prepared, and equal protein concentrations were separated using Criterion Tris-HCl gels (Bio-Rad). Proteins were then transferred to polyvinylidene difluoride membranes and blocked for 1 h at room temperature with nonfat milk containing 0.1% Tween 20. Membranes were then incubated overnight in primary antibodies diluted in blocking solution. Membranes were washed in PBS containing 0.1% Tween 20 and incubated in horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Protein bands were visualized by chemiluminescence.
Measurement of Nitrite—Nitrite, a stable breakdown product of nitric oxide, was measured in cell medium using the Griess reaction. Briefly, 100 μl of conditioned medium was mixed with 50 μl each of Griess reagent R1 and R2 (Cayman Chemical) in a 96-well plate, and absorbance was measured at 542 nm.
Sodium Borohydride Reduction and C18 SepPak Purification of Synthetic A4-NP or Oxidized DHA—0.5 mg of oxDHA or 100 μgofA4-NP was adjusted to pH 3, extracted with ethyl acetate, dried, and resuspended in 1 ml of methanol. 1 ml of 12% (w/w) sodium borohydride (NaBH4) in water was added to the methanol and incubated on ice for 30 min. Samples were diluted in 20 ml of water and adjusted to pH 3 and then subjected to solid phase extraction using a C18 SepPak. Briefly, the SepPak column was washed with 7 ml of methanol and 10 ml of pH 3 water, and then the sample was added, followed by washes with 10 ml of pH 3 water and 10 ml of heptane. The samples were eluted with 10 ml of 1:1 ethyl acetate/heptane and dried under nitrogen and resuspended in ethanol at appropriate stock concentrations.
Preparation of A4-NP and oxDHA-Glutathione Conjugates—A4-NP or oxDHA were reacted with GSH in the presence of glutathione S-transferase (GST) as described previously (19). Briefly, 0.5 mg of oxDHA or 100 μgofA4-NP were added to 1 ml of PBS containing 1 mg of GSH and 1 mg of rat liver GST (Sigma) and incubated at 37 °C for 2 h. Samples were then diluted in 10 ml of pH 3 water and subjected to C18 SepPak purification as described above.
Human Brain Samples—Post-mortem human cortical brain samples were provided by T. Montine from the University of Washington brain bank. Control and AD patients were matched for age and smoking status, and samples were obtained, frozen, and stored as described previously (20). A4/J4-NPs were extracted from brain tissue and hydrolyzed from phospholipids with Apis mellifera phospholipase A2 as described previously (21).
Purification and Gas Chromatography (GC)/Mass Spectrometry (MS) Analysis of Unesterified A4/J4-NPs—A4/J4-NPs were quantified by GC/MS as described previously (14). Briefly, samples were spiked with 20 ng of 2H4-PGA2 internal standard then extracted using C18 Sep-Pak cartridges as described above. The eluted samples were converted to O-methyloxime pentafluorobenzyl ester derivatives, purified by thin layer chromatography employing a solvent system of hexane/acetone (70:30, v/v), converted to trimethylsilyl ether derivatives, and quantified by stable isotope dilution techniques employing GC/MS on an Agilent 6890N GC/MS instrument (Santa Clara, CA). The major ions generated in the NICI mass spectra of the pentafluorobenzyl ester, O-methyloxime, trimethylsilyl ether derivatives of A4/J4 -NPs, and the 2H4-PGA2 are the carboxylate anions at m/z 458 and m/z 438, respectively.
RESULTS
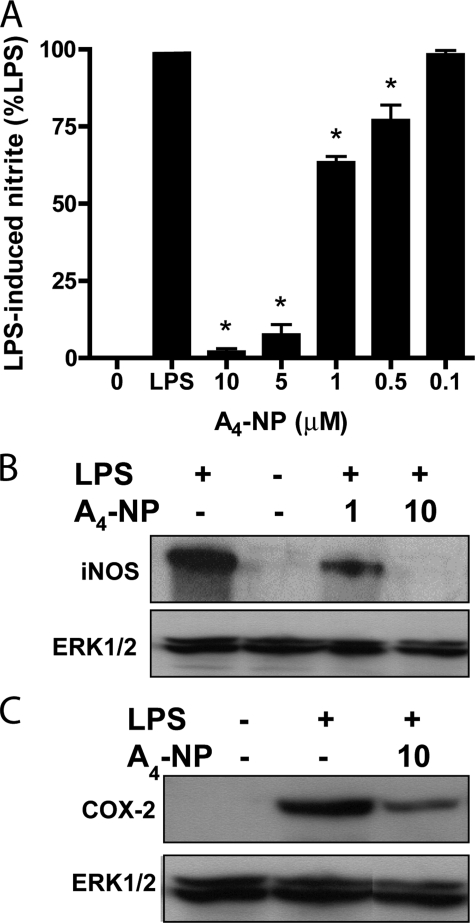
A4-NP Inhibits LPS-induced iNOS and COX-2 Expression in RAW Macrophages—Although A4/J4-NPs are formed abundantly in vivo and are structurally similar to anti-inflammatory cyclopentenone PGs (Fig. 1), the cellular effects of A4/J4-NPs have never been explored. We obtained synthetic A4-NP, an endogenous cyclopentenone NP, to examine the bioactivity of this class of DHA metabolites. We found that synthetic A4-NP potently suppressed LPS-induced nitric oxide production (as measured by accumulation of nitrite in cell media) by RAW264.7 macrophages in a dose-dependent manner with an IC50 ∼ 2 μm (Fig. 2A). This was not because of cytotoxicity, as 10 μm A4-NP did not alter RAW cell viability, as assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (data not shown). A4-NP also inhibited LPS-stimulated expression of the pro-inflammatory proteins iNOS and COX-2 (Fig. 2, B and C). Suppression of iNOS expression was more complete than COX-2 expression, an effect also noted with cyclopentenone IsoPs, which is thought to be due to regulation of COX-2 expression by several pathways other than NF-κB (22).
FIGURE 2.
A4-NP inhibits LPS-induced production of inflammatory mediators in macrophages. A, RAW macrophages were pretreated with vehicle (0) or A4-NP for 30 min and then stimulated with LPS (1 μg/ml) for 10 h, at which time cell media were subjected to nitrite assay. No nitrite was detectable in unstimulated cells (1st 0 bar), and data were normalized to nitrite produced by LPS alone (LPS). A4-NP inhibits LPS-induced nitrite production with an IC50 ∼ 2 μm. Graph represents mean ± S.E. from at least three independent experiments. *, p < 0.05 versus LPS alone. B and C, RAW cells were treated as in A and then harvested at 6 h and subjected to Western blot analysis employing antibodies to iNOS (B) and COX-2 (C). ERK1/2 is shown as a loading control, and blots are representative of three independent experiments.
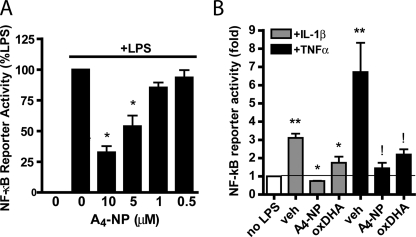
A4-NP Is an Inhibitor of the NF-κB Pathway—Because expression of iNOS and COX-2 is known to be regulated by the transcription factor NF-κB (22, 23), we examined the effect of A4-NP on NF-κB signaling. NF-κB transcriptional activity was assessed using immortalized mouse macrophages expressing an NF-κB-responsive luciferase reporter. As shown in Fig. 3A, A4-NP inhibited LPS-induced NF-κB-mediated transcription in a dose-dependent manner. A4-NP also completely blocked NF-κB activation induced by the pro-inflammatory cytokines TNFα and IL-1β (Fig. 3B). LPS, TNFα, and IL-1β activate NF-κB via distinct receptors and signaling pathways, which converge at the level of IκB kinase complex (IKK) activation (8). This finding demonstrates that A4-NP-mediated inhibition of NF-κB signaling does not occur at the receptor level.
FIGURE 3.
A4-NP inhibits NF-κB transcriptional activity induced by multiple stimuli. A, NF-κB reporter macrophages were pretreated for 30 min with vehicle or A4-NP and then stimulated with LPS for 4 h, at which time luciferase assays were performed. Data are expressed as % LPS-induced luciferase increase, as LPS increased luciferase production between 4- and 7-fold depending on experiment. B, NF-κB reporter macrophages were treated as in A but were stimulated with IL-1β (20 ng/ml) or TNFα (20 ng/ml). 10 μm A4-NP and 30 μm oxDHA were employed. Data are expressed as fold increase above unstimulated cells. Data represent mean ± S.E. of three independent experiments. *, p < 0.05 versus LPS alone (A) or TNFα alone (B). **, p < 0.05 versus no LPS, !, p < 0.05 versus IL-1β alone.
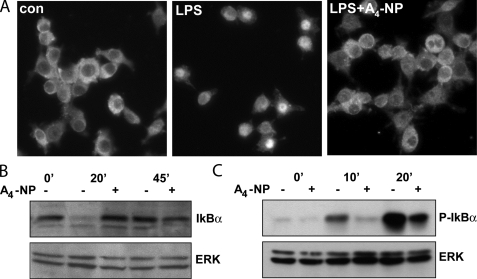
Inhibition of NF-κB Signaling by A4-NP Occurs at the Level of IKK Function—Next, we sought to determine how A4-NP inhibits NF-κB signaling. NF-κB-mediated transcription requires translocation of p50/p65 heterodimers from the cytosol to the nucleus. Immunostaining of RAW264.7 cells demonstrated NF-κB p65 confined to the cytoplasm, whereas LPS stimulation resulted in intense nuclear p65 accumulation after 30 min (Fig. 4A). Co-treatment with A4-NP blocked LPS-induced p65 nuclear translocation.
FIGURE 4.
A4-NP inhibits NF-κB signaling at the level of IKK function. A, RAW cells were pretreated with vehicle (con) or A4-NP (10 μm) for 30 min and then stimulated with LPS for 30 min. Cells were then fixed and stained for NF-κB p65 subunit, which was visualized by fluorescence microscopy. LPS causes a pronounced nuclear accumulation of p65 that is blocked by A4-NP. B, RAW cells were treated as in A, then harvested at indicated time points, and subjected to Western blot analysis with anti-IκBα antibody. A4-NP prevents LPS-induced IκBα degradation. C, RAW cells were treated as in A, and the proteasome inhibitor MG132 was added 5 min prior to LPS stimulation. Lysates were subjected to Western blot analysis with a phospho-IκBα Ser-32/36 antibody. ERK blots are shown as loading controls. Images and blots are representative of three independent experiments.
LPS-stimulated nuclear translocation of p65 is dependent on the phosphorylation and subsequent proteasomal degradation of IκBα, an inhibitor protein that associates with the p65/p50 heterodimer and holds it in the cytoplasm. Western blot analysis demonstrated marked degradation of IκBα after 20 min of LPS treatment, whereas A4-NP blocked LPS-induced IκBα degradation at the same time point (Fig. 4B). Similar results were obtained in TNFα-treated mouse macrophages (data not shown).
Phosphorylation of IκBα at Ser-32 and -36 by IKK precedes its dissociation from p65/p50 and proteasomal degradation. To examine IκBα phosphorylation, RAW cells were preincubated with A4-NP or vehicle for 30 min and then stimulated with LPS and treated with the proteasome inhibitor MG132 (40 μm), so as to prevent IκBα degradation. Phosphorylation of IκBα was detected by Western blot with a Ser-32/36 phospho-specific antibody. LPS caused pronounced IκBα phosphorylation at 10 min, which was almost completely abrogated by A4-NP (Fig. 4C). A4-NP also substantially inhibited IκBα phosphorylation after 20 min of LPS exposure. These results, coupled with the ability of A4-NP to suppress NF-κB activation triggered by TNFα and IL-1β, suggest that A4-NP may inhibit NF-κB signaling at the level of the IKK complex.
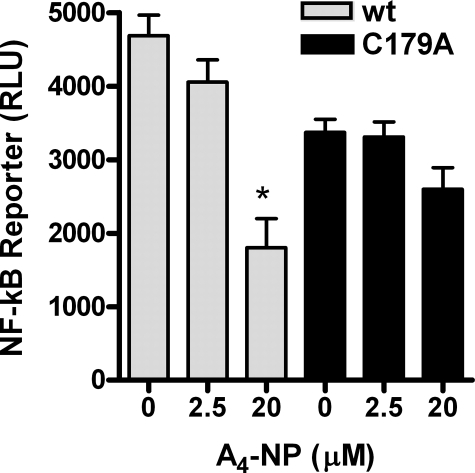
Mutation of IKKβ Cysteine 179 Impairs the Ability of A4-NPs to Inhibit NF-κB Signaling—We next sought to determine whether A4-NPs inhibit NF-κB activation by perturbation of IKK function. Cys-179 in IKKβ contains a thiol group susceptible to Michael adduction, and cyclopentenone PGs are known to inhibit NF-κB signaling via adduction of this residue (15). Thus, we examined the impact of mutation of IKKβ Cys-179 to alanine, which prevents Michael adduct formation at that site, on the action of A4-NP on NF-κB activation. As shown in Fig. 5, overexpression of WT or mutant C179A IKKβ caused a marked increase in NF-κB-luc reporter activity in HEK293 cells in the absence of LPS signaling. A4-NP suppressed this increase significantly by a mean of 62% in WT IKKβ cells (p < 0.05) but did not significantly decrease NF-κB activation in C179A mutant cells, demonstrating that A4-NPs inhibit IKK activity at least in part via modification of Cys-179.
FIGURE 5.
Mutation of IKKβ cysteine 179 abrogates the effect of A4-NP on NF-κB activation. HEK293 cells were transfected with WT (gray bars) or mutant (C179A, black bars) IKKβ expression vectors and NF-κB-luc reporter construct and then treated with A4-NP for 8 h to allow time for endogenous turnover of luciferase, at which time cells were harvested and assayed for NF-κB-luc activity. Control cells were transfected with empty vector and NF-κB-luc reporter, and the base-line luciferase activity from these cells was subtracted as background from IKK-transfected cells. *, p < 0.05 versus untreated cells. Figure represents data from three separate experiments.
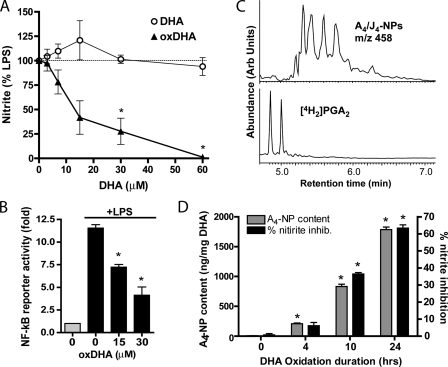
Oxidation of DHA Increases A4/J4-NP Content and Anti-inflammatory Potency in Parallel—Because synthetic A4-NP inhibits NF-κB signaling, we next sought to examine the formation of A4/J4-NPs during DHA oxidation, and correlate this with the anti-inflammatory effect of DHA. As shown in Fig. 6A, treatment with unoxidized DHA did not affect LPS-induced nitrite production in RAW cells in the acute setting (30 min of preincubation), whereas oxidized DHA (which had been subjected to 10 h of oxidation with 5 mm AAPH) caused a dose-dependent inhibition of nitric oxide production. oxDHA also inhibited LPS-stimulated NF-κB reporter activity at similar concentrations in immortalized murine macrophages (Fig. 6B). oxDHA also inhibits NF-κB activation by IL-1β or TNFα (Fig. 3B). oxDHA subjected to solid phase extraction on a C18 SepPak, which removes unoxidized DHA from the mixture but preserves A4/J4-NPs, retained its anti-inflammatory potency, suggesting that oxidation products, and not residual DHA, are indeed responsible for this effect.
FIGURE 6.
A4/J4-NPs are formed abundantly during DHA oxidation as assessed by GC/MS and their formation correlates with anti-inflammatory potency. A, oxidized DHA (oxDHA) was generated via oxidation for 10 h in 5 mm AAPH. RAW macrophages were pretreated with unoxidized DHA (DHA) or oxDHA for 30 min and then stimulated with LPS (1 μg/ml) for 10 h, at which point nitrite assay was performed. B, NF-κB reporter macrophages were treated with oxDHA as in A and then stimulated with LPS for 4 h, at which point luciferase assays were performed. Data are expressed as fold increase in luciferase production over unstimulated cells (gray bar). *, p < 0.05 versus LPS alone. C, selected ion current GC/MS chromatogram obtained from the analysis of A4/J4-NPs generated during oxidation of DHA in vitro. The two large peaks in the m/z 438 ion current chromatogram represent the syn- and anti-O-methyloxime isomers of the [2H4]PGA2 internal standard. The series of peaks in the m/z 458 chromatogram represent A4/J4-NPs. The amount of A4/J4-NPs represented is 1738 ng/mg DHA. D, aliquots of DHA were oxidized in 5 mm AAPH for increasing duration and then analyzed for A4/J4-NP content by GC-MS (gray bars, left y axis). These same aliquots were applied to RAW cells at a concentration of 10 μm using the same methods as in Fig. 2A, and their ability to suppress LPS-induced nitrite production was assessed (black bars, right y axis). *, p < 0.05 versus time point 0 (unoxidized DHA). All experiments were carried out in triplicate, and data represent mean ± S.E.
We next employed a stable isotope dilution GC/MS method with a 4H2-PGA2 internal standard to quantify A4/J4-NPs in oxDHA (14). As shown in Fig. 6C, we identified a series of peaks using selected ion monitoring with an m/z ratio of 458, which elute similarly to the 4H2-PGA2 internal standard and represent A4/J4-NPs. Quantification of these peaks revealed that A4/J4-NPs were abundant in DHA subjected to 24 h of oxidation (1785 ± 76 ng/mg of DHA), in keeping with previous reports (14).
We next examined the relationship between A4/J4-NP formation and the anti-inflammatory potency of DHA. DHA aliquots were oxidized for increasing durations and then subjected to mass spectrometric analysis. As shown in Fig. 6D, the content of A4/J4-NPs increased markedly with longer oxidation duration. Interestingly, the anti-inflammatory potency of these aliquots increased in almost exact parallel with A4/J4-NP content (Fig. 6D).
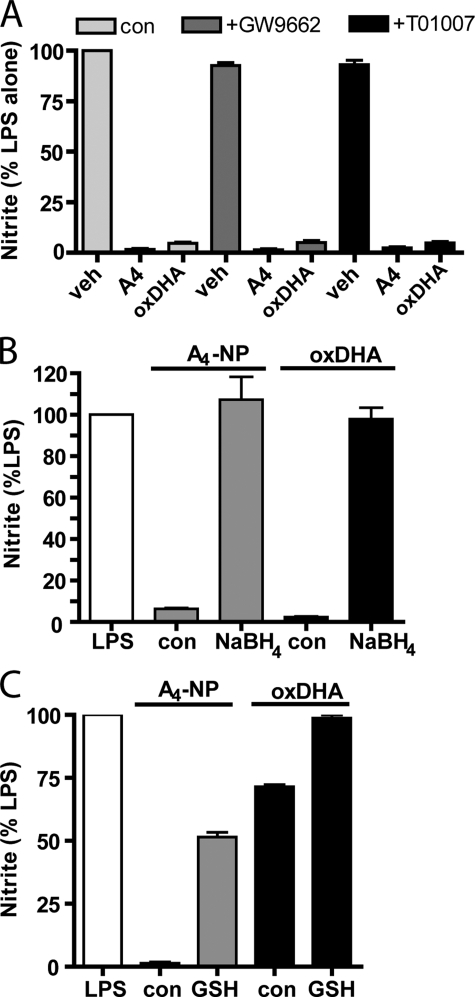
Bioactivity of Both A4-NPs and oxDHA Is Independent of PPARγ and Is Eliminated by Chemical Reduction or Conjugation to GSH—Several reports have implicated activation of the PPARγ nuclear receptor in the anti-inflammatory effects of cyclopentenone molecules and of DHA (24, 25). However, we observed that two molecularly distinct PPARγ receptor antagonists, GW9662 and T0070907, used at concentrations well above their respective IC50 values, failed to inhibit the anti-inflammatory effects of A4-NP (Fig. 7A), suggesting that PPARγ is not crucially involved. We also observed that PPARγ antagonists had no effect on the anti-inflammatory effect of oxDHA (Fig. 7A).
FIGURE 7.
The anti-inflammatory effect of A4-NP or oxDHA is independent of PPARγ and can be abrogated by reduction with NaBH4 or adduction to glutathione. A, RAW cells were treated with vehicle or one of two PPARγ antagonists (GW9662, 1 μm, or T01007, 500 nm) for 30 min, then pretreated with A4-NP (10 μm) or oxDHA (40 μm), and stimulated with LPS as in previous experiments. p < 0.05 versus LPS alone. B and C, A4-NP (gray bars, 10 μm) or oxDHA (black bars, 40 μm in B and 10 μm in C) was subjected to reduction with NaBH4 (B) or incubated with GST and GSH (+GSH) for 2 h (C) and then purified by C18 SepPak and applied to RAW cells 30 min prior to LPS stimulation as in previous experiments. Control A4-NP and oxDHA was subjected to identical incubation and purification in the absence of NaBH4 or GSH. C18 SepPak purification removes residual GST, GSH, and NaBH4, as well as unoxidized DHA. Graphs in B and C are from a single experiment performed in triplicate and are representative of results from three independent experiments.
Many of the biological effects of cyclopentenone eicosanoids are because of their ability to form Michael adducts with thiol groups in key intracellular proteins (26). To assess this possibility, A4-NP was subjected to chemical reduction with NaBH4, a reductant that reduces the carbonyl moiety on the cyclopentenone ring to a nonreactive alcohol. As shown in Fig. 7B, chemical reduction completely abrogated the anti-inflammatory effect of A4-NP, whereas A4-NP subjected to the identical incubation and purification steps in the absence of NaBH4 retained its bioactivity. As we have implicated A4-NPs as an active component of oxDHA, we next examined the effect of NaBH4 reduction on the bioactivity of oxDHA. Like A4-NP, exposure of oxDHA to NaBH4 completely abrogated the anti-inflammatory effect of oxDHA (Fig. 7B). Utilizing MS approaches, we also determined that NaBH4-treated oxDHA resulted in the disappearance of chromatographic peaks shown in Fig. 6C and appearance of peaks at an m/z ratio 2 Da higher suggesting that the cyclopentenone moiety has been reduced to an alcohol (data not shown). These data suggest that molecules containing reducible ketone moieties, potentially cyclopentenone-containing compounds, contribute to the bioactivity of oxDHA.
Cyclopentenone-containing eicosanoids are metabolized via conjugation with GSH by GSTs, yielding biologically inactive GSH conjugates (19, 27). Our group has shown previously that exposure of cyclopentenone isoprostanes to GST and GSH yields GSH conjugates that no longer possess anti-inflammatory activities (28). We found similarly that incubation of A4-NP with GSH and bovine liver GST substantially inhibited its anti-inflammatory effects (Fig. 7C). Treatment of oxDHA with GST and GSH, which would presumably inactivate any cyclopentenone-containing molecules or other reactive lipids via formation of inactive glutathione conjugates, also blocked the anti-inflammatory effect of the oxDHA mixture (Fig. 7C). It should be noted that residual GSH, GST, and NaBH4 were removed by C18 column purification prior to cell treatment.
Incubation of RAW cells with 10 μm A4-NP for 1 h also did not alter cellular levels of GSH (data not shown), demonstrating that A4-NPs mechanism of action is not reliant on the depletion of intracellular GSH. Taken together, these results suggest that the bioactivity of A4-NP is mediated largely by its chemical reactivity and ability to form thiol adducts with proteins, and are consistent with a role for cyclopentenone NPs in the anti-inflammatory effect of oxDHA, although the inactivation of other reactive compounds present in the oxDHA mixture cannot be ruled out.
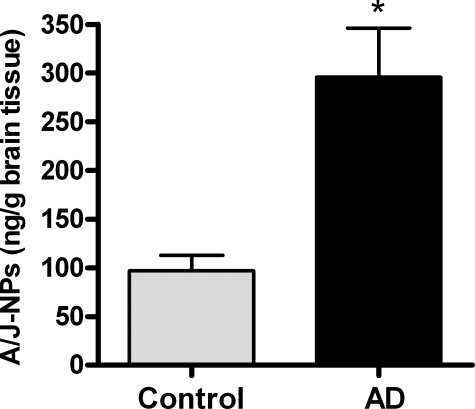
A4/J4-NPs Are Found Abundantly in Human Brain Specimens from Patients with Alzheimer Disease—The formation of A4/J4-NPs has never been previously demonstrated in human disease states. Alzheimer disease (AD) is characterized by progressive and extensive neuroinflammation, as well as oxidative injury and lipid peroxidation (29, 30). As neural tissue is rich in DHA, previous studies have demonstrated increased levels of nonreactive F4-NPs in AD brain samples (20). We obtained frontal cortex brain samples from pathologically confirmed AD and age-matched control patients and examined levels of A4/J4-NPs by GC/MS. As shown in Fig. 8, esterified levels of A4/J4-NPs were ∼3-fold greater in AD brain than controls. The average level of A4/J4-NPs in an AD brain sample was 295 ng/g brain tissue, which roughly converts to 950 nm A4/J4-NPs. This finding is important in that 500 nm A4-NP exerted significant anti-inflammatory effects in our cell culture system, showing that A4/J4-NPs are found in biologically relevant concentrations in humans in vivo.
FIGURE 8.
A4/J4-NPs levels are increased in post-mortem brain samples from patients with Alzheimer disease. Post-mortem frontal cortex samples were obtained from patients with pathologically confirmed AD (n = 6) and age-matched controls (n = 6). Levels of phospholipids-esterified A4/J4-NPs were quantified by GC/MS. *, p < 0.01 versus control.
DISCUSSION
Although the anti-inflammatory benefits of the ω-3 fatty acid DHA are well described, it is unclear if DHA itself, or a DHA metabolite, mediates these effects. Here we have demonstrated that a novel family of cyclopentenone docosanoids termed A4/J4-NPs, derived from the peroxidation of DHA, suppress the inflammatory response in macrophages via inhibition of NF-κB signaling. One A4/J4-NP available to us in chemically pure form as a racemic mixture, A4-NP, potently inhibits iNOS and COX-2 expression in stimulated macrophages by a mechanism that involves blockade of NF-κB signaling through inhibition of IKK function via cysteine modification. Furthermore, we show that oxidation of DHA significantly enhances the anti-inflammatory potency of this fatty acid, and that quantities of A4/J4-NPs formed correlate very closely with the anti-inflammatory potency of oxDHA. Chemical reduction or conjugation to glutathione, both of which render A4/J4-NPs biologically inert, also abrogates the anti-inflammatory effects of oxDHA. Taken together, these results identify A4/J4-NPs as novel anti-inflammatory mediators derived from DHA and reveal that free radical-mediated peroxidation of DHA and subsequent formation of A4/J4-NPs may well contribute to the anti-inflammatory effects of this ω-3 fatty acid.
NPs are generated abundantly in vivo via nonenzymatic free radical-mediated oxidation of DHA. The mechanism of NP formation involves the generation of several possible endoperoxide intermediates, each of which can undergo reduction or rearrangement to yield different ring structures. Thus, NPs are present as racemic mixtures of stereo- and regioisomers. Furthermore, they are generated with different functional groups on the cyclopentane ring. Reducing conditions favor the formation of stable F-ring NPs (F4-NPs), which can be quantified as an index of membrane lipid peroxidation (13, 31). Under other conditions, for example when cellular levels of GSH or α-to-copherol are depleted, the formation of D- and E-ring NPs is favored. These latter compounds rapidly dehydrate to yield A4/J4-NPs both in vitro and in vivo (14, 32). Indeed, we have previously reported that A4/J4-NPs are readily detectable in rat tissues. Here we extend those findings to demonstrate that A4/J4-NPs are formed in human brain and are increased to biologically relevant concentrations in Alzheimer disease, a condition associated with oxidative stress, inflammation, and DHA oxidation (30).
Several cyclopentenone prostanoids (including 15-deoxy Δ12,14-PGJ2 and 15-J2-IsoP) have been reported to inhibit NF-κB activity through activation of PPARγ, although this does not appear to be the case for A4-NP (24, 26, 28, 33). We have found that the acute anti-inflammatory effects of A4-NP are independent of PPARγ and are likely reliant on thiol adduction, as reduction of the molecule with NaBH4 or conjugation to GSH, both of which eliminate the reactivity of this molecule, abrogate the effects of A4-NP. Furthermore, nonreactive F4-NPs, which are structurally similar to A4-NP except that they contain hydroxyl groups on the ring as opposed to a reactive unsaturated carbonyl structure, do not inhibit NF-κB.3 Our results also show that A4-NP inhibits IKK-dependent IκBα phosphorylation, and that this effect is abrogated by mutation of cysteine 179 in the activation loop of IKKβ. Previous studies have reported that the cyclopentenone PG, PGA2, can directly inhibit IKK function via adduction of this thiol (15). Thus, it seems that the mechanism of action of A4-NP is similar to that of a number of endogenous electrophilic lipids, including cyclopentenone PGs and IsoPs and 4-hydroxynonenal, all of which inhibit NF-κB signaling primarily through thiol modification and inhibition of protein function (15, 26, 34).
Numerous anti-inflammatory mechanisms of action have been proposed to explain the beneficial effects of DHA supplementation, including activation of PPAR-γ (25), alteration of intracellular redox signaling (7), and inhibition of TLR4 (35), AKT (35), lipid rafts (36), protein kinase Cζ, and NADPH oxidase (37). However, in these studies, it is unclear whether DHA itself, or a DHA metabolite, is the active component. Various oxidized products of DHA have recently been reported that possess significant biological activities. For example, Serhan and co-workers have described lipoxygenase-derived DHA metabolites, termed protectins and D-series resolvins, which exert potent anti-inflammatory effects and contribute to the resolution of inflammation (9, 38). These compounds are generated by the action of lipoxygenases on DHA, and it is unclear to what extent they are formed by nonenzymatic oxidation. It is very likely that other oxidized lipid species and mechanisms contribute to the many biological actions of DHA. The contribution of each mechanism is also likely highly dependent on the products generated as well as the experimental conditions utilized. The studies examining the biological effects of A4/J4-NPs reported herein focus on acute inflammation, and cells were exposed to A4-NPs or oxDHA for a short period of time prior to the inflammatory stimulus. Other reports have utilized much longer preincubation schemes, which may involve different signaling mechanisms other than NF-κB (6, 37).
Our results reported herein are consistent with the conclusion that cyclopentenone NPs contribute to the anti-inflammatory effects of DHA. The finding that reduction of oxDHA with NaBH4, like that reported with A4-NP, abrogates its anti-inflammatory activity is important because it suggests that our results with oxDHA cannot be explained by the presence of hydroxylated DHA derivatives (such as protectins and resolvins), as NaBH4 would not be expected to alter the structure of these alcohols. The ability of GSH conjugation to eliminate oxDHA bioactivity also is consistent with a role for A4/J4-NPs, and eliminates from consideration many classes of peroxidation products that do not react with GSH. Identification of the active molecules in DHA is crucial for the optimization of ω-3-based therapies, and would allow for standardization of fish oil regimens in clinical trials.
DHA has demonstrated therapeutic efficacy in a number of inflammation-related diseases (1). As an example, dietary supplementation with ω-3 fatty acids, in particular DHA, decreases risk of AD, and protects against neuronal damage in animal models of AD (39–41). Neuroinflammation plays a key role in AD pathogenesis, and nitric oxide elaboration by activated microglia appears to contribute to neuronal death in this disease (42). Accordingly, genetic deletion of iNOS is protective in mouse AD models (43). Thus, it is reasonable to postulate that the suppression of iNOS expression and nitric oxide by A4/J4-NPs may underlie some of the protective effects of fish oil in AD, although this hypothesis has not yet been tested. Levels of D4/E4-NPs, precursors to A4/J4-NPs, are elevated in affected brain regions from AD patients (20). We have found that A4/J4-NPs are also present in human brain and are elevated to biologically relevant levels in Alzheimer disease. One would expect that supplementation with DHA might increase A4-NP levels to concentrations at which they could exert anti-inflammatory effects and have a potential protective benefit.
In summary, we examined the biological activity of cyclopentenone NPs using chemically synthesized A4-NP and show that it is a potent inhibitor of inflammation in macrophages acting via inhibition of NF-κB-mediated signaling. Furthermore, we have demonstrated that A4/J4-neuroprostanes present in oxDHA are anti-inflammatory mediators formed endogenously via free radical-mediated oxidation of this fatty acid and may account for some of the anti-inflammatory effects of DHA. Thus, we suggest that the formation of some lipid peroxidation products, such as A4/J4-NPs, may not be a detrimental biological process, and that the susceptibility of DHA to oxidation may in fact be a virtue rather than a vice in some cases. A more thorough understanding of the mechanisms of action of DHA, and the oxidized lipid species that mediate these actions, could lead to the development of targeted strategies that afford even greater therapeutic potential for this ω-3 fatty acid.
Acknowledgments
We thank Dr. M. Karin for generously providing the IKKβ C179A plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grants GM15431, CA77839, DK48831, and ES13125. This work was also supported by the Italian MIUR and Regione Lombardia, Direzione Generale Sanità. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; NP, neuroprostane; PG, prostaglandin; IsoP, isoprostane; iNOS, inducible nitric-oxide synthase; LPS, lipopolysaccharide; COX-2, cyclooxygenase-2; AAPH, 2,2′-azobis-(2-amidinopropane) hydrochloride; NF-κB, nuclear factor κB; TNFα, tumor necrosis factor-α; IL-1β, interleukin 1β; PPAR, peroxisome proliferator-activated receptor; IκBα, inhibitor of κBα; IKK, IκBα kinase; oxDHA, oxidized DHA; A4-NP, 14-A4-NP; GC, gas chromatography; MS, mass spectrometry; DAPI, 4′,6-diamidno-2-phenylindole; GST, glutathione S-transferase; AD, Alzheimer disease; PBS, phosphate-buffered saline; ERK, extracellular signal-related kinase; WT, wild type.
E. Musiek, unpublished observations.
References
- 1.Calder, P. C. (2006) Am J. Clin. Nutr. 83 Suppl. 6, 1505–1519 [Google Scholar]
- 2.Mori, T. A., and Beilin, L. J. (2004) Curr. Atheroscler. Rep. 6 461–467 [DOI] [PubMed] [Google Scholar]
- 3.Belluzzi, A., Brignola, C., Campieri, M., Pera, A., Boschi, S., and Miglioli, M. (1996) N. Engl. J. Med. 334 1557–1560 [DOI] [PubMed] [Google Scholar]
- 4.Weldon, S. M., Mullen, A. C., Loscher, C. E., Hurley, L. A., and Roche, H. M. (2007) J. Nutr. Biochem. 18 250–258 [DOI] [PubMed] [Google Scholar]
- 5.Volker, D., Fitzgerald, P., Major, G., and Garg, M. (2000) J. Rheumatol. 27 2343–2346 [PubMed] [Google Scholar]
- 6.De Caterina, R., Cybulsky, M. I., Clinton, S. K., Gimbrone, M. A., Jr., and Libby, P. (1994) Arterioscler. Thromb. Vasc. Biol. 14 1829–1836 [DOI] [PubMed] [Google Scholar]
- 7.Komatsu, W., Ishihara, K., Murata, M., Saito, H., and Shinohara, K. (2003) Free Radic. Biol. Med. 34 1006–1016 [DOI] [PubMed] [Google Scholar]
- 8.Karin, M., and Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18 621–663 [DOI] [PubMed] [Google Scholar]
- 9.Marcheselli, V. L., Hong, S., Lukiw, W. J., Tian, X. H., Gronert, K., Musto, A., Hardy, M., Gimenez, J. M., Chiang, N., Serhan, C. N., and Bazan, N. G. (2003) J. Biol. Chem. 278 43807–43817 [DOI] [PubMed] [Google Scholar]
- 10.Sun, Y. P., Oh, S. F., Uddin, J., Yang, R., Gotlinger, K., Campbell, E., Colgan, S. P., Petasis, N. A., and Serhan, C. N. (2007) J. Biol. Chem. 282 9323–9334 [DOI] [PubMed] [Google Scholar]
- 11.Porter, N. A., Caldwell, S. E., and Mills, K. A. (1995) Lipids 30 277–290 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez, M. J., Gray, J. I., Schemmel, R. A., Dugan, L., Jr., and Welsch, C. W. (1992) J. Nutr. 122 2190–2195 [DOI] [PubMed] [Google Scholar]
- 13.Roberts, L. J., II, Montine, T. J., Markesbery, W. R., Tapper, A. R., Hardy, P., Chemtob, S., Dettbarn, W. D., and Morrow, J. D. (1998) J. Biol. Chem. 273 13605–13612 [DOI] [PubMed] [Google Scholar]
- 14.Fam, S. S., Murphey, L. J., Terry, E. S., Zackert, W. E., Chen, Y., Gao, L., Pandalai, S., Milne, G. L., Roberts, L. J., Porter, N. A., Montine, T. J., and Morrow, J. D. (2002) J. Biol. Chem. 277 36076–36084 [DOI] [PubMed] [Google Scholar]
- 15.Rossi, A., Kapahi, P., Natoli, G., Takahashi, T., Chen, Y., Karin, M., and Santoro, M. G. (2000) Nature 403 103–108 [DOI] [PubMed] [Google Scholar]
- 16.Straus, D. S., Pascual, G., Li, M., Welch, J. S., Ricote, M., Hsiang, C. H., Sengchanthalangsy, L. L., Ghosh, G., and Glass, C. K. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 4844–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanoni, G., Brunoldi, E. M., Porta, A., and Vidari, G. (2007) J. Org. Chem. 72 9698–9703 [DOI] [PubMed] [Google Scholar]
- 18.Blackwell, T. S., Yull, F. E., Chen, C. L., Venkatakrishnan, A., Blackwell, T. R., Hicks, D. J., Lancaster, L. H., Christman, J. W., and Kerr, L. D. (2000) Am J. Resp. Crit. Care Med. 162 1095–1101 [DOI] [PubMed] [Google Scholar]
- 19.Milne, G. L., Zanoni, G., Porta, A., Sasi, S., Vidari, G., Musiek, E. S., Freeman, M. L., and Morrow, J. D. (2004) Chem. Res. Toxicol. 17 17–25 [DOI] [PubMed] [Google Scholar]
- 20.Reich, E. E., Markesbery, W. R., Roberts, L. J., II, Swift, L. L., Morrow, J. D., and Montine, T. J. (2001) Am. J. Pathol. 158 293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow, J. D., Minton, T. A., Mukundan, C. R., Campbell, M. D., Zackert, W. E., Daniel, V. C., Badr, K. F., Blair, I. A., and Roberts, L. J., II (1994) J. Biol. Chem. 269 4317–4326 [PubMed] [Google Scholar]
- 22.Chun, K. S., and Surh, Y. J. (2004) Biochem. Pharmacol. 68 1089–1100 [DOI] [PubMed] [Google Scholar]
- 23.Xie, Q. W., Kashiwabara, Y., and Nathan, C. (1994) J. Biol. Chem. 269 4705–4708 [PubMed] [Google Scholar]
- 24.Chawla, A., Barak, Y., Nagy, L., Liao, D., Tontonoz, P., and Evans, R. M. (2001) Nat. Med. 7 48–52 [DOI] [PubMed] [Google Scholar]
- 25.Li, H., Ruan, X. Z., Powis, S. H., Fernando, R., Mon, W. Y., Wheeler, D. C., Moorhead, J. F., and Varghese, Z. (2005) Kidney Int. 67 867–874 [DOI] [PubMed] [Google Scholar]
- 26.Perez-Sala, D., Cernuda-Morollon, E., Pineda-Molina, E., and Canada, F. J. (2002) Ann. N. Y. Acad. Sci. 973 533–536 [DOI] [PubMed] [Google Scholar]
- 27.Atsmon, J., Freeman, M. L., Meredith, M. J., Sweetman, B. J., and Roberts, L. J. N. (1990) Cancer Res. 50 1879–1885 [PubMed] [Google Scholar]
- 28.Musiek, E. S., Gao, L., Milne, G. L., Han, W., Everhart, M. B., Wang, D., Backlund, M. G., DuBois, R. N., Zanoni, G., Vidari, G., Blackwell, T. S., and Morrow, J. D. (2005) J. Biol. Chem. 280 35562–35570 [DOI] [PubMed] [Google Scholar]
- 29.Wyss-Coray, T. (2006) Nat. Med. 12 1005–1015 [DOI] [PubMed] [Google Scholar]
- 30.Montine, T. J., and Morrow, J. D. (2005) Am J. Pathol. 166 1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musiek, E. S., Cha, J. K., Yin, H., Zackert, W. E., Terry, E. S., Porter, N. A., Montine, T. J., and Morrow, J. D. (2004) J. Chromatogr. B 799 95–102 [DOI] [PubMed] [Google Scholar]
- 32.Reich, E. E., Zackert, W. E., Brame, C. J., Chen, Y., Roberts, L. J., II, Hachey, D. L., Montine, T. J., and Morrow, J. D. (2000) Biochemistry 39 2376–2383 [DOI] [PubMed] [Google Scholar]
- 33.Ricote, M., Li, A. C., Willson, T. M., Kelly, C. J., and Glass, C. K. (1998) Nature 391 79–82 [DOI] [PubMed] [Google Scholar]
- 34.Ji, C., Kozak, K. R., and Marnett, L. J. (2001) J. Biol. Chem. 276 18223–18228 [DOI] [PubMed] [Google Scholar]
- 35.Lee, J. Y., Ye, J., Gao, Z., Youn, H. S., Lee, W. H., Zhao, L., Sizemore, N., and Hwang, D. H. (2003) J. Biol. Chem. 278 37041–37051 [DOI] [PubMed] [Google Scholar]
- 36.Fan, Y. Y., Ly, L. H., Barhoumi, R., McMurray, D. N., and Chapkin, R. S. (2004) J. Immunol. 173 6151–6160 [DOI] [PubMed] [Google Scholar]
- 37.Massaro, M., Habib, A., Lubrano, L., Del Turco, S., Lazzerini, G., Bourcier, T., Weksler, B. B., and De Caterina, R. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwab, J. M., Chiang, N., Arita, M., and Serhan, C. N. (2007) Nature 447 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris, M. C., Evans, D. A., Bienias, J. L., Tangney, C. C., Bennett, D. A., Wilson, R. S., Aggarwal, N., and Schneider, J. (2003) Arch. Neurol. 60 940–946 [DOI] [PubMed] [Google Scholar]
- 40.Schaefer, E. J., Bongard, V., Beiser, A. S., Lamon-Fava, S., Robins, S. J., Au, R., Tucker, K. L., Kyle, D. J., Wilson, P. W., and Wolf, P. A. (2006) Arch. Neurol. 63 1545–1550 [DOI] [PubMed] [Google Scholar]
- 41.Calon, F., Lim, G. P., Yang, F., Morihara, T., Teter, B., Ubeda, O., Rostaing, P., Triller, A., Salem, N., Jr., Ashe, K. H., Frautschy, S. A., and Cole, G. M. (2004) Neuron 43 633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGeer, E. G., and McGeer, P. L. (1998) Exp. Gerontol. 33 371–378 [DOI] [PubMed] [Google Scholar]
- 43.Nathan, C., Calingasan, N., Nezezon, J., Ding, A., Lucia, M. S., La Perle, K., Fuortes, M., Lin, M., Ehrt, S., Kwon, N. S., Chen, J., Vodovotz, Y., Kipiani, K., and Beal, M. F. (2005) J. Exp. Med. 202 1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]