Abstract
Resveratrol may protect against metabolic disease through activating SIRT1 deacetylase. Because we have recently defined AMPK activation as a key mechanism for the beneficial effects of polyphenols on hepatic lipid accumulation, hyperlipidemia, and atherosclerosis in type 1 diabetic mice, we hypothesize that polyphenol-activated SIRT1 acts upstream of AMPK signaling and hepatocellular lipid metabolism. Here we show that polyphenols, including resveratrol and the synthetic polyphenol S17834, increase SIRT1 deacetylase activity, LKB1 phosphorylation at Ser428, and AMPK activity. Polyphenols substantially prevent the impairment in phosphorylation of AMPK and its downstream target, ACC (acetyl-CoA carboxylase), elevation in expression of FAS (fatty acid synthase), and lipid accumulation in human HepG2 hepatocytes exposed to high glucose. These effects of polyphenols are largely abolished by pharmacological and genetic inhibition of SIRT1, suggesting that the stimulation of AMPK and lipid-lowering effect of polyphenols depend on SIRT1 activity. Furthermore, adenoviral overexpression of SIRT1 stimulates the basal AMPK signaling in HepG2 cells and in the mouse liver. AMPK activation by SIRT1 also protects against FAS induction and lipid accumulation caused by high glucose. Moreover, LKB1, but not CaMKKβ, is required for activation of AMPK by polyphenols and SIRT1. These findings suggest that SIRT1 functions as a novel upstream regulator for LKB1/AMPK signaling and plays an essential role in the regulation of hepatocyte lipid metabolism. Targeting SIRT1/LKB1/AMPK signaling by polyphenols may have potential therapeutic implications for dyslipidemia and accelerated atherosclerosis in diabetes and age-related diseases.
AMPK (AMP-activated protein kinase)2 serves as a sensor of cellular energy status, being activated by increased AMP/ATP ratio or by the upstream kinases, LKB1 (the tumor suppressor kinase), CaMKKβ (Ca2+/calmodulin-dependent protein kinase kinase β), and TAK1 (transforming growth factor-β-activated kinase-1) (1–7). Our previous studies demonstrated that dysfunction of hepatic AMPK induced by hyperglycemia represents a key mechanism for hepatic lipid accumulation and hyperlipidemia associated with diabetes (8, 9). Also, metformin, an antidiabetic drug, lowers systemic and hepatic lipids via activating LKB1/AMPK signaling (2, 8, 10). Our recent studies with human hepatocytes and type 1 diabetic LDL receptor-deficient (LDLR–/–) mice have shown that polyphenols strongly stimulate hepatic AMPK and reduce lipid accumulation, which in turn attenuates hyperlipidemia and atherosclerosis in diabetic mice (9). Therefore, AMPK activation by polyphenols or metformin may be at least partially responsible for their therapeutic benefits on hyperlipidemia in diabetes (2, 8, 9). Resveratrol also stimulates AMPK in neurons (11). However, rapid activation of AMPK by polyphenols has been shown to be independent of altered adenine nucleotide levels (9, 11). Also, resveratrol activates AMPK in intact cells via an indirect mechanism, since it does not activate AMPK in a cell-free assay (12). The signaling molecules that mediate the metabolic actions of AMPK activation by polyphenols are poorly understood.
SIRT1, a mammalian ortholog of Sir2 (silent information regulator 2), is an NAD-dependent deacetylase that acts as a master metabolic sensor of NAD+ and modulates cellular metabolism and life span (13–15) and delays the onset of age-related diseases (16). SIRT1 has been implicated in the control of energy metabolism through deacetylation of FOXO and PGC-1α (proliferator-activated receptor γ coactivator 1) (13, 17, 18). The impact of SIRT1 on diabetes is evidenced by the fact that newly discovered small molecule activators of SIRT1 improve insulin sensitivity in type 2 diabetes (19). Resveratrol, a putative mimetic of caloric restriction, increases mitochondrial biogenesis and insulin sensitivity through SIRT1 activation (12, 19–21). Interestingly, the insulin sensitivity and mitochondrial activity are also regulated by AMPK (22, 23). Because some of the beneficial metabolic actions of polyphenols are mediated by their ability to activate SIRT1 (12, 20) or AMPK (9), we hypothesized that polyphenols may protect against high glucose-induced lipid accumulation in hepatocytes by activating SIRT1 and AMPK.
The aim of the present study is to test whether SIRT1 is a critical regulator of AMPK signaling in controlling hepatocellular lipid metabolism. We show here that polyphenols, including resveratrol and 6,8-diallyl-5,7-dihydroxy-2-(2-allyl-3-hydroxy-4-methoxyphenyl)1-H benzo(b)pyran-4-one (S17834), potently increase both SIRT1 deacetylase activity and AMPK activity, which in turn reduces lipid accumulation in HepG2 hepatocytes exposed to high glucose. Also, these responses to polyphenols are dependent on SIRT1. Moreover, adenoviral overexpression of SIRT1 increases the basal AMPK activity in HepG2 cells and in mouse liver. SIRT1 also suppresses expression of FAS (fatty acid synthase) and lipid accumulation through activating AMPK. Furthermore, AMPK activation by polyphenol-activated SIRT1 is mediated by the upstream kinase, LKB1, but not CaMKKβ. Therefore, SIRT1 activation by polyphenols functions as an upstream regulator in the LKB1/AMPK signaling axis. Because of the associated improvements of hyperlipidemia and atherosclerosis observed in type 1 or type 2 diabetic mice treated with polyphenols (9, 12, 20), these findings suggest that targeting SIRT1/LKB1/AMPK signaling by polyphenols may have potential therapeutic implications for lipid metabolic disorders and accelerated atherosclerosis in diabetes and age-related diseases.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies—The SIRT1 activity assay kits (catalog number AK-555) were purchased from BIOMOL (Plymouth Meeting, PA). Adenopure™ kits for adenovirus purification were from Puresyn Inc. (Malvern, PA). Infinity™ triglyceride reagents were from Thermo DMA (Louisville, CO). Resveratrol and splitomicin were obtained from Calbiochem, nicotinamide was from Sigma, and STO-609 (CaMKK inhibitor) was from Tocris Bioscience (Ellisville, MO). S17834, a synthetic polyphenol, was previously described (9) and was provided by the Institut de Recherches Servier (Suresnes, France). Rabbit polyclonal phospho-Thr172 AMPKα, phospho-Ser428 LKB1 antibodies, total AMPKα and ACC antibodies were purchased from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal phospho-Ser79 ACC1 (Ser221 ACC2) antibody and Sir2 antibody were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Rabbit polyclonal AMPKα1 or -α2 isoform antibodies were from Bethyl Laboratories Inc. (Montgomery, TX). Rabbit polyclonal anti-SIRT1 antibody (sc-15404), mouse monoclonal LKB1 antibody (sc-32245), and horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse monoclonal antibodies against FAS (catalog number 610962), CaMKK (catalog number 610544), and anti-Myc (9E10) antibody were from BD Biosciences. Mouse monoclonal anti-FLAG M2 antibody was from Sigma. Mouse monoclonal anti-β-actin antibody was from Abcam Inc. (Cambridge, MA).
SIRT1 Deacetylase Activity Assays—The Fluor de Lys fluorescence assay for in vitro SIRT1 activity (24) was performed by incubation with recombinant human SIRT1 and substrates, including a fluorogenic acetylated Lys382 p53 peptide (50 μm) and NAD (100 μm) at 37 °C for 30 min according to the BIOMOL manufacturer's instructions. Fluorescent intensity was measured using a Fluoroskan Ascent® microplate fluorometer (Thermo Electron Corp., Milford, MA). Negative controls included “No Enzyme” and “Time Zero” controls, in which Developer II solution plus 2 mm nicotinamide was added before mixing the substrates with or without the SIRT1 enzyme. SIRT1 activity was calculated with the corrected arbitrary fluorescence units of the tested compounds to “no enzyme” control and expressed as fluorescent units relative to the control. To rule out whether polyphenolic compounds possess autofluorescence or nicotinamide itself interferes with the fluorescent signal, the Developer II solution was incubated with the Fluor de Lys deacetylated standard or the tested compounds in the absence of SIRT1 enzyme or substrates. The deacetylated standard dose-dependently increased the fluorescent rate, whereas the tested compounds did not alter the fluorescent intensity, indicating the change in p53 deacetylation caused by these compounds depends on specific SIRT1 activity (data not shown).
Cell Culture and Treatments—Human HepG2 hepatocytes, human embryonic kidney (HEK) 293 cells, and HeLa cells that lack LKB1 (American Type Culture Collection, Manassas, VA) were cultured in DMEM containing 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin, and 5.5 mm d-glucose (normal glucose), as previously described (8, 9, 25). To characterize the functional relevance of SIRT1 to AMPK signaling and lipid metabolism, high glucose-induced lipid accumulation in HepG2 hepatocytes model was studied (8, 9, 26, 27).
Adenoviruses and Cell Infection—Adenoviral vectors encoding the wild type mouse SIRT1 (Ad-SIRT1) (28) were a generous gift from Dr. Sadoshima (New Jersey Medical School, Newark, NJ). Adenoviral vectors encoding FLAG-tagged wild type mouse SIRT1 (Ad-FLAG-SIRT1), an inactive mutant of SIRT1 with a histidine 355 to alanine mutation (Ad-SIRT1H355A), and Ad-SIRT1 short hairpin RNA (shRNA) and Ad-control shRNA (15, 17, 18, 28) were kindly provided by Drs. Puigserver and Rodgers (Harvard Medical School, Boston, MA). Adenoviral vector expressing FLAG-tagged wild type LKB1 (Ad-FLAG-LKB1) was kindly provided by Dr. Walsh (Boston University School of Medicine, Boston, MA). Adenoviral vectors encoding green fluorescent protein (GFP) and a Myc-tagged dominant-negative mutant of AMPKα2 (Ad-DN-AMPK, AMPKα K45R) were described elsewhere (8, 9). The replication-deficient adenoviruses were amplified in HEK293 cells and purified by Adenopure™ kits. Cells were infected with adenoviruses for 36–48 h prior to the experiments as described elsewhere (8, 9).
Lentivirus-mediated SIRT1 shRNA—Lentivirus expressing short hairpin RNA (shRNA) for human SIRT1 was generated as previously described (8, 25, 29, 30). For knockdown of human SIRT1, the sequence GTATTGCTGAACAGATGGAA was chosen for the shRNA target. The expression cassette was created by tandem PCR with human U6 promoter as a template using CACCGCGCGCCAAGGTCGGGCA for forward primer and CTACACAAACTCCAcCTGTTCAGCAATACGGTGTTTCGTCC as the first reverse primer and CCAAAAAAGTATTGCTGAACAGATGGAACTACACAAACTC as the second reverse primer. The produced expression cassette was inserted into a directional pENTR/D-topo vector (Invitrogen) and then transferred to shRNA expressing lentivirus vector pDSL_hpUGIP (ATCC) by LR-clonase (Invitrogen). Recombinant lentiviruses were produced by co-infection into HEK293T cells with the lentivirus plasmid and three other helper vectors, pLP-1, pLP-2, and pVSVG (Invitrogen), using the calcium phosphate method. Lentiviral supernatant was harvested at 72 h postinfection and filtered through a 0.45-μm membrane. HepG2 cells were infected with fresh lentivirus expressing either control shRNA or SIRT1 shRNA in DMEM containing 8 μg/ml Polybrene (Sigma) for 24 h and cultured for an additional 72 h. The cells were selected for puromycin resistance (0.6 μg/ml, 7 days).
Immunoblot Analysis—Immunoblotting of cell lysates was carried out according to our previous experimental procedure (8, 9, 25, 31). The phosphorylation of LKB1, AMPK, and ACC was analyzed by immunoblots with antibodies against phospho-Ser428 LKB1, phospho-Thr172 AMPK, and phospho-Ser79 ACC1 (Ser221 ACC2), which are specific AMPK phosphorylation sites, as well as total LKB, AMPKα1 or -α2, or ACC as loading controls. In some cases, we used AMPKα2 as a loading control because our previous studies showed similar expression levels of endogenous AMPKα1 or -α2 protein in HepG2 cells and in the mouse livers (9). The levels of phosphorylation were quantified by scanning densitometry using a model GS-700 imaging densitometer (Bio-Rad), normalized to the levels of total protein and expressed as relative phosphorylation to the basal or control level. In some cases, two bands of phosphorylated and total ACC were detected in HepG2 cells and in the mouse livers, which was also observed by other studies (32, 33), and phosphorylation intensity of ACC was expressed as the ratio of the sum of the two bands of phosphorylated ACC to the sum of the two bands of endogenous ACC. In addition, expression of FAS (∼270 kDa) was assessed by immunoblots with FAS antibody, and its levels were normalized to those of β-actin and presented as the -fold change relative to the control.
Measurement of Hepatocellular Triglyceride Content—Intracellular triglyceride contents were measured in HepG2 cell lysates, as previously described (8, 9, 34, 35). To assay, 30 μl of triglyceride standard or cleared cell supernatant was added to a 96-well flat bottom polystyrene plate, and 300 μl of Infinity triglyceride reagent was then added to the microplate. After the plate was incubated for 5 min, the optical density was read at 520 nm with a SPECTRAmax340 Microplate Spectrophotometer (Molecular Devices Corp.). Intracellular triglyceride levels were normalized to protein concentrations and expressed as μg of lipid/mg of protein (8, 9).
In Vivo Adenoviral Gene Transfer—All of the animal experiments were performed according to the guidelines for the Care and Use of Laboratory Animals of Boston University. Male C57BL/6 mice at 10 weeks of age received standard mouse chow diet and water ad libitum. Mice were kept under isoflurane anesthesia during surgery. One hundred microliters containing 5 × 109 to 1 × 1010 plaque-forming units per mouse of Ad-FLAG-SIRT1 or Ad-GFP were injected through the right jugular vein using a 0.1-ml syringe with a 29.5-gauge needle (36). Mice were sacrificed at 7 days postinjection, and livers were immediately dissected and flash-frozen in liquid nitrogen. To assess the expression of recombinant SIRT1 and the phosphorylation of AMPK and ACC in the liver, ∼100 mg of frozen liver was placed in 1 ml of lysis buffer (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 1% (v/v) Nonidet P-40, 1 mm EDTA, 1 mm EGTA, 1 mm sodium orthovanadate, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 1 μg/ml pepstatin) and homogenized on the ice (9). Samples were allowed to stand on ice for 30 min and then centrifuged at 14,000 rpm at 4 °C for 15 min, and the supernatant was analyzed for immunoblots.
Statistical Analysis—All data are presented as the means ± S.E. Statistical analysis was performed by a two-tailed unpaired Student's t test. A value of p < 0.05 was accepted as statistically significant.
RESULTS
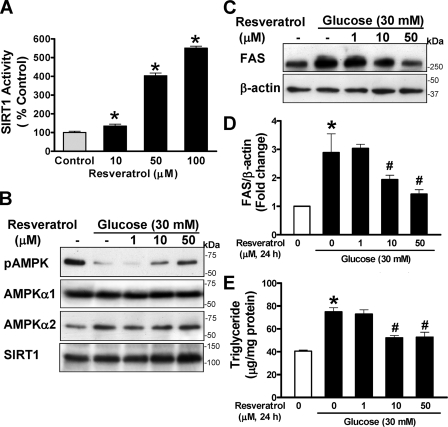
Pharmacological Activation of SIRT1 by Polyphenols Stimulates AMPK and Prevents Enhanced Expression of FAS and Elevated Lipid Levels Caused by High Glucose—To test the hypothesis that activation of SIRT1 by polyphenols is connected to the modulation of AMPK signaling, the effect of resveratrol and a synthetic polyphenol, S17834, on recombinant SIRT1 deacetylase activity was assessed. SIRT1 deacetylation activity was slightly but significantly increased by resveratrol as low as 10 μm and displayed a 4–5-fold increase at higher concentrations (50–100 μm) (Fig. 1A), consistent with the results of earlier studies (24). S17834 at 50 μm also caused a ∼2-fold increase in SIRT1 activity (data not shown). These results indicate that SIRT1 deacetylase is activated by the two polyphenolic compounds.
FIGURE 1.
Pharmacological activation of SIRT1 by resveratrol prevents the decrease in AMPK activity and elevation in FAS expression and triglyceride level in human HepG2 hepatocytes exposed to high glucose. A, the dose-response effect of resveratrol on in vitro SIRT1 deacetylase activity. Fluor de Lys fluorescence deacetylase assays were preformed with human recombinant SIRT1, using a synthetic acetylated Lys382 p53 peptide and NAD as substrates in the absence or presence of increasing concentrations (10–100 μm) of resveratrol as described under “Experimental Procedures.” SIRT1 activity was expressed as arbitrary fluorescence units relative to the control (mean ± S.E., n = 4). *, p < 0.05 versus control. B, resveratrol prevents the inhibition of AMPK caused by high glucose in a dose-dependent manner. HepG2 cells were maintained in serum-free DMEM containing normal glucose overnight and incubated for 24 h without or with increasing concentrations (1–50 μm) of resveratrol in the absence or presence of 30 mm d-glucose (high glucose). Representative immunoblotting analysis with antibodies against AMPKα phosphorylated at Thr172 (pAMPK) and total AMPKα1 or -α2 for loading controls, respectively, is shown. Immunoblots with anti-SIRT1 antibody show the existence of ∼120 kDa of endogenous SIRT1 in HepG2 cells and no detectable change in SIRT1 throughout treatment. C and D, high glucose increases and resveratrol suppresses expression of FAS. Expression of FAS was analyzed by immunoblots with anti-FAS and normalized to β-actin level and presented as the -fold change (mean ± S.E., n = 3). E, resveratrol protects against high glucose-induced triglyceride accumulation. Intracellular triglyceride contents were measured and expressed as μg of lipid/mg of protein (the mean ± S.E., n = 4) as described under “Experimental Procedures.” *, p < 0.05 versus normal glucose; #, p < 0.05 versus high glucose alone.
We previously demonstrated that polyphenols strongly and persistently stimulate AMPK activity more potently than metformin (9). As shown in supplemental Fig. 1, A and B, phosphorylation of AMPK was stimulated 2-fold over the basal level by resveratrol at 50 μm and sustained up to ∼3.5-fold at 100 μm and was associated with a dose-dependent increase in ACC phosphorylation in human HepG2 hepatocytes. Consistently, resveratrol (50 μm) increased the phosphorylation of AMPK and ACC to an extent similar to that of specific AMPK α1 and α2 isoform kinase activity as measured by a SAMS peptide assay (9). The results demonstrate that resveratrol (10–100 μm) strongly stimulates both SIRT1 deacetylase and AMPK activity in a concentration-dependent manner.
In the liver, glucose induces the expression of genes involved in lipogenesis, such as FAS, a key enzyme that catalyzes de novo fatty acid synthesis from glucose, leading to increased incorporation of free fatty acid into triglyceride (37). To determine whether activation of SIRT1 and AMPK by polyphenols is of functional relevance, HepG2 cells were treated with resveratrol (1–50 μm, 24 h) in the presence of high glucose, and AMPK, ACC, FAS, and lipid levels were determined. As shown in Fig. 1, B–E, and supplemental Fig. 1, C and D, impaired AMPK signaling caused by high glucose was associated with a 2.8-fold increase in FAS expression and consequently ∼2-fold elevation in intracellular triglyceride content, as was seen in hepatic lipid accumulation in diabetic mice (9). In contrast, decreased AMPK and ACC phosphorylation caused by high glucose was counteracted by resveratrol at 10 or 50 μm, reaching a level comparable with that in untreated cells. Notably, altered AMPK activity caused by resveratrol or by high glucose could be attributed to their ability to regulate SIRT1 activity, since SIRT1 expression was not significantly changed throughout treatments. Activation of SIRT1 by resveratrol also dose-dependently reversed enhanced FAS expression and elevated triglyceride level caused by high glucose, which is associated with its ability to activate AMPK. Resveratrol strongly stimulated AMPK activity and inhibited lipid accumulation caused by high glucose to an extent similar to that of S17834 (9). The results suggest that SIRT1 activation by polyphenols stimulates AMPK and ACC phosphorylation, which in turn inhibits ACC activity as well as FAS expression, thereby reducing the triglyceride accumulation.
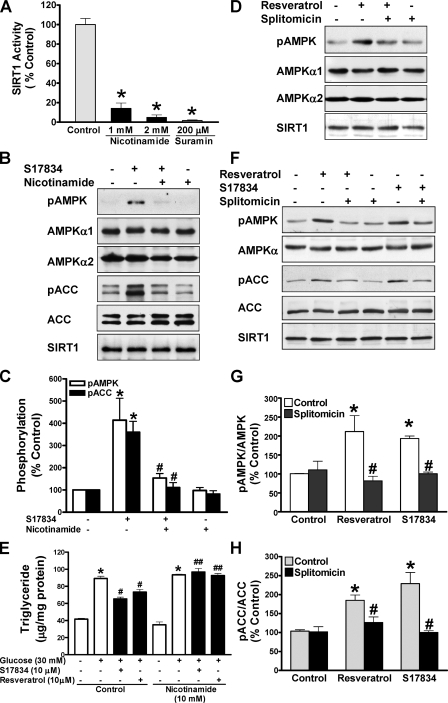
Pharmacological Inhibition of SIRT1 Attenuates Polyphenol-induced AMPK Activation and Lipid Reduction—To further characterize the potential role of SIRT1 in regulating AMPK and its functional consequences, we examined the influence of pharmacological SIRT1 inhibitors on AMPK signaling and lipids. Consistent with it being a product and inhibitor of the deacetylase reaction (13), nicotinamide at 1 or 2 mm caused an 80% decrease in SIRT1-mediated deacetylation of p53 (Fig. 2A). As shown in Fig. 2, B and C, when HepG2 cells were pretreated with nicotinamide at 10 mm, a concentration that was previously shown to significantly decrease deacetylation of histones in cardiac myocytes (28), and then treated with S17834 (10 μm), phosphorylation of AMPK and ACC was enhanced ∼4-fold by S17834, and the increase was largely blocked by nicotinamide, returning to a nearly normal level. Nicotinamide completely blocked the lipid-lowering effect of resveratrol or S17834 in HepG2 cells incubated in high glucose, although it did not alter the basal lipid levels (Fig. 2E). The results demonstrate that polyphenols stimulate AMPK activity and lower lipids in a nicotinamide-sensitive manner.
FIGURE 2.
Pharmacological inhibition of SIRT1 attenuates polyphenol-induced AMPK activation and lipid reduction in human HepG2 cells or HEK293 cells. A, SIRT1 deacetylase activity is largely inhibited by nicotinamide. The SIRT1 activity assay was carried out in the absence or presence of nicotinamide or suramin, an SIRT1 inhibitor included in the SIRT1 activity assay kit. *, p < 0.05 versus control. B–D, inhibition of SIRT1 activity by nicotinamide or splitomicin diminishes enhanced phosphorylation of AMPK and ACC in response to the polyphenol in HepG2 cells. Cells were pretreated without or with either nicotinamide (10 mm) or splitomicin (100 μm) in the serum-free medium for 24 h and then incubated without or with S17834 (10 μm) or resveratrol (50 μm) for an another 1 h. Densitometric quantification of the phosphorylation of AMPK and ACC is shown. *, p < 0.05 versus control; #, p < 0.05 versus polyphenol alone (mean ± S.E., n = 3). E, nicotinamide prevents the lipid-lowering effect of polyphenols in HepG2 cells. Cells were pretreated for 24 h without or with nicotinamide (10 mm) in serum-free medium and incubated for 24 h without or with polyphenols in the presence of high glucose. *, p < 0.05 versus normal glucose alone; #, p < 0.05 versus high glucose alone; ##, p < 0.05 versus high glucose plus polyphenols (mean ± S.E., n = 4). F–H, the polyphenols increase and splitomicin decreases phosphorylation of AMPK and ACC in HEK293 cells. Phosphorylation of AMPK and ACC was assessed in cells pretreated with splitomicin (100μm) for 24 h and incubated with resveratrol (50 μm) or S17834 (10 μm) for an additional 1 h as indicated. *, p < 0.05 versus control; #, p < 0.05 versus polyphenol alone (mean ± S.E., n = 3). No detectable change in the expression of endogenous SIRT1 was observed throughout treatments in both human cell lines.
Next, the effect of splitomicin, a selective and cell-permeable inhibitor of SIRT1 (38), was determined. Like nicotinamide, activation of AMPK by resveratrol was markedly diminished by splitomicin in HepG2 cells (Fig. 2D). Similarly, resveratrol (50 μm) significantly increased AMPK and ACC phosphorylation ∼2-fold as effectively as S17834 (10 μm), and the response to polyphenols was almost completely prevented by splitomicin in HEK293 cells (Fig. 2, F–H). These data indicate that the effect of activators or inhibitors of SIRT1 to modulate AMPK signaling occurs in different cell types.
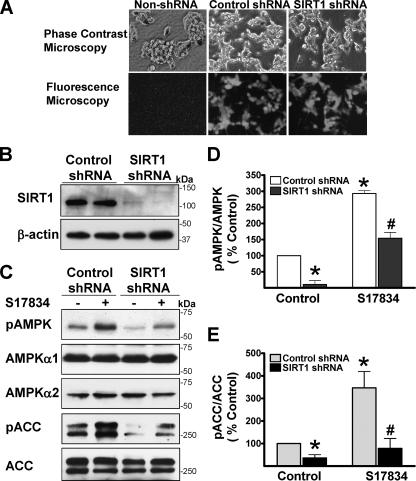
The Ability of Polyphenols to Stimulate AMPK and Reduce Lipid Accumulation Is Abrogated by Knockdown or Inhibition of SIRT1—We rigorously examined the involvement of SIRT1 in regulating AMPK by using several independent approaches to knock down or inhibit SIRT1. Lentiviral vectors expressing either an shRNA control or shRNA targeting human SIRT1 were infected into HepG2 cells. As shown in Fig. 3, compared with control shRNA, expression of endogenous SIRT1 protein (∼120 kDa) was remarkably suppressed by SIRT1 shRNA, but no detectable change in other proteins, such as AMPKα1 or -α2 and ACC, was observed, suggesting that the knockdown effect of this SIRT1 shRNA is specific. Notably, cells infected with SIRT1 shRNA grew more slowly than cells expressing control shRNA. No difference in the basal and polyphenol-stimulated phosphorylation of AMPK and ACC was seen between non-lentivirus-infected cells and control shRNA-infected cells (data not shown). Importantly, compared with control shRNA, knockdown of SIRT1 by shRNA reduced the basal phosphorylation of AMPK and ACC by ∼70%, suggesting that the basal AMPK activity is largely dependent on the presence of SIRT1. Moreover, enhanced phosphorylation of AMPK by the polyphenol was remarkably attenuated by specific SIRT1 knockdown, and consequently S17834-induced phosphorylation of ACC was almost completely blocked, reaching nearly the basal level seen in control shRNA.
FIGURE 3.
Lentivirus-mediated knockdown of SIRT1 diminishes the basal and polyphenol-induced AMPK activation in HepG2 cells. HepG2 cells were infected without or with lentivirus expressing either an shRNA control or SIRT1 shRNA and then selected in 0.6 μg/ml puromycin. Cells were allowed to recover from the selection for 1 week prior to the experiments. A, co-expression of GFP in both control shRNA cells and SIRT1 shRNA cells was observed under fluorescence microscopy. B, endogenous SIRT1 expression was largely suppressed by lentivirus expressing SIRT1 shRNA. Representative immunoblots for the expression of SIRT1 and β-actin are shown in duplicates under the identical condition. C–E, knockdown of SIRT1 by lentivirus-mediated SIRT1 shRNA down-regulates the basal and polyphenol-stimulated AMPK and ACC phosphorylation. HepG2 cells expressing either control or SIRT1 shRNA were quiesced in serum-free medium overnight and treated with S17834 (10 μm, 1 h). *, p < 0.05 versus untreatment in cells expressing control shRNA; #, p < 0.05 versus S17834 treatment in cells expressing control shRNA (mean ± S.E., n = 3).
To exclude off-target effects of SIRT1 shRNA, an adenovirus expressing SIRT1 shRNA, which up-regulates the acetylation of PGC-1 and FOXO, two known targets of SIRT1, in mouse livers (17, 18), was used to assess the effect of resveratrol on AMPK signaling. Compared with Ad-control shRNA, expression of endogenous SIRT1 and resveratrol-induced phosphorylation of AMPK was consistently decreased by Ad-SIRT1 shRNA, without changes in the expression of AMPKα2 (supplemental Fig. 2). Taken together, these results indicate that SIRT1 positively regulates AMPK signaling, and polyphenol-stimulated AMPK activation at least partially depends on SIRT1.
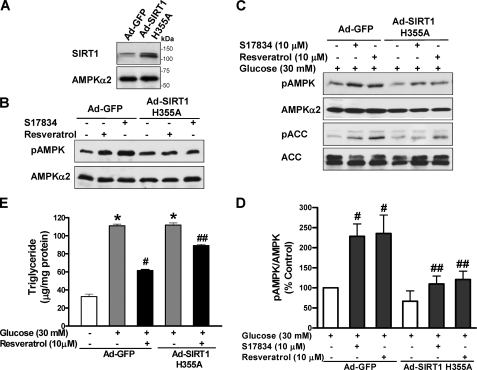
To seek further evidence for the functional relevance of SIRT1-stimulated AMPK activation to its regulation of lipid metabolism, HepG2 cells were infected with an adenoviral vector encoding a catalytically inactive mutant of SIRT1 (Ad-SIRT1H355A), which was shown to enhance the NAD-dependent acetylation of p53 and PGC-1α, two well characterized substrates of SIRT1 (17, 28, 39, 40). As shown in Fig. 4, A and B, in cells expressing Ad-GFP, resveratrol or S17834 rapidly and potently stimulated AMPK phosphorylation, and this effect was remarkably abrogated by overexpression of a SIRT1H355A mutant (∼120 kDa) that was confirmed by immunoblots with SIRT1 antibody. The results also demonstrate that deacetylation activity of SIRT1 plays a key role in mediating polyphenol-stimulated AMPK activation. Moreover, as shown in Fig. 4, C–E, in Ad-GFP-infected cells under conditions of high glucose, phosphorylation of AMPK and ACC was stimulated ∼2-fold by either resveratrol or S17834. In contrast, SIRT1H355A-infected cells exhibited significantly less phosphorylation than control GFP cells. Furthermore, chronic AMPK activation by resveratrol caused a 50% reduction in triglyceride levels that were elevated by high glucose in Ad-GFP-infected cells, but the SIRT1H355A mutant attenuated the lipid-lowering effect of polyphenols. Taken together, our findings with pharmacological and genetic inhibition of SIRT1 indicate that polyphenols stimulate AMPK and suppress lipid accumulation at least in part through SIRT1 activation.
FIGURE 4.
Polyphenol-induced AMPK activation and lipid reduction is abolished by overexpression of a catalytically inactive mutant of SIRT1 (SIRT1H355A) in HepG2 cells. A, a representative immunoblot of overexpression of an adenovirus vector encoding a catalytically inactive SIRT1 mutant in HepG2 cells is shown. B, polyphenol-stimulated AMPK phosphorylation is abolished by the SIRT1H355A mutant under normal glucose conditions. HepG2 cells were infected for 48 h with Ad-GFP or Ad-SIRT1H355A, followed by treatment with S17834 (10 μm) or resveratrol (50 μm) for 1 h. C and D, polyphenol-stimulated AMPK signaling is diminished by the SIRT1H355A mutant under high glucose conditions. HepG2 cells infected with Ad-GFP or Ad-SIRT1H355A were quiesced in serum-free medium overnight and treated for 24 h without or with 10 μm of resveratrol or S17834 in the presence of high glucose. E, the lipid-lowering effect of resveratrol is attenuated by the SIRT1H355A mutant. *, p < 0.05 versus normal glucose in cells expressing GFP; #, p < 0.05 versus high glucose alone in cells expressing GFP; ##, p < 0.05 versus polyphenol treatment in cells expressing GFP (mean ± S.E., n = 4).
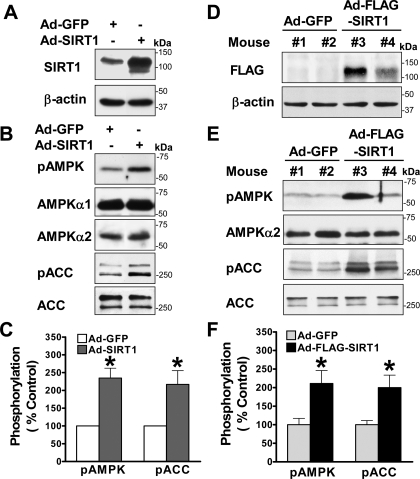
Overexpression of SIRT1 Stimulates Hepatocyte AMPK Signaling in Vitro and in Vivo—To gain direct evidence for the role of hepatocyte SIRT1 in modulating AMPK, we examined whether adenovirus-mediated overexpression of wild type SIRT1 (Ad-SIRT1) (28) could stimulate AMPK signaling in HepG2 cells. As shown in Fig. 5, A–C, phosphorylation of AMPK was stimulated ∼2-fold over the basal level by Ad-SIRT1. Consistently, overexpression of SIRT1 led to an ∼2-fold increase in ACC phosphorylation. The increase in the basal AMPK activity by SIRT1 mimicked that of resveratrol at 50 μm (supplemental Fig. 1B) and expression of a constitutively active AMPK (8). Thus, a role for SIRT1 in stimulating AMPK is further supported by the evidence that both overexpression and knockdown of SIRT1 are sufficient to modulate the basal AMPK activity.
FIGURE 5.
Hepatic overexpression of SIRT1 is sufficient to enhance phosphorylation of AMPK and ACC over the basal levels in HepG2 cells and in mouse liver in vivo. A, immunoblots with SIRT1 antibody confirm overexpression of recombinant SIRT1 protein (∼120 kDa) in HepG2 cells infected with adenovirus-mediated vector encoding wild type SIRT1 (Ad-SIRT1). B and C, overexpression of SIRT1 is sufficient to increase the baseline phosphorylation of AMPK and ACC in HepG2 cells. D, in vivo expression of adenovirus-mediated vector encoding FLAG-tagged wild type SIRT1 (Ad-FLAG-SIRT1) is visualized by Western blots with anti-FLAG antibody in the livers from different C57BL/6 mice that were sacrificed 7 days postinjection as indicated. Ad-GFP-infected mice were used as a control. E and F, phosphorylation of AMPK and ACC is increased in the livers of mice injected with Ad-FLAG-SIRT1 in vivo. Representative immunoblots of phosphorylation of AMPK and ACC in the livers from two mice each group as indicated and densitometric analysis are shown. *, p < 0.05 versus Ad-GFP-infected HepG2 cells or Ad-GFP-injected mice (mean ± S.E., n = 3).
Because adenovirus-mediated overexpression of SIRT1 specifically in mouse liver was recently shown to lower hepatic lipids by down-regulation of SREBP (sterol regulatory element-binding protein), a lipogenic transcription factor, and its target gene, FAS (18), we further investigated the in vivo effects of overexpression of SIRT1 on AMPK signaling in the liver of C57BL/6 mice. Adenoviral SIRT1 gene transfer was accomplished by jugular vein injection of a recombinant FLAG-tagged wild type SIRT1 (Ad-FLAG-SIRT1) (17). Although the immunoblot with anti-SIRT1 antibody can detect endogenous human SIRT1 in cultured HepG2 and HEK293 cells (Fig. 2), it does not detect endogenous mouse SIRT1 and the Ad-FLAG-tagged SIRT1 expressed in mouse livers. We performed the immunoblot with anti-FLAG antibody to confirm that the FLAG-tagged SIRT1 was expressed in the livers of mice injected with Ad-FLAG-SIRT1 but not in those of control mice injected with Ad-GFP (Fig. 5D). Thus, hepatic expression of recombinant FLAG-SIRT1 was successfully achieved in mice in vivo. Furthermore, hepatic overexpression of SIRT1 caused an ∼2-fold increase in the basal phosphorylation of AMPK and ACC in vivo (Fig. 5, E and F), with no detectable changes in endogenous AMPKα or ACC protein. These in vitro and in vivo studies define a novel role of hepatic SIRT1 in stimulating the basal AMPK activity, which may explain the inhibitory effect of SIRT1 on hepatic lipids (18).
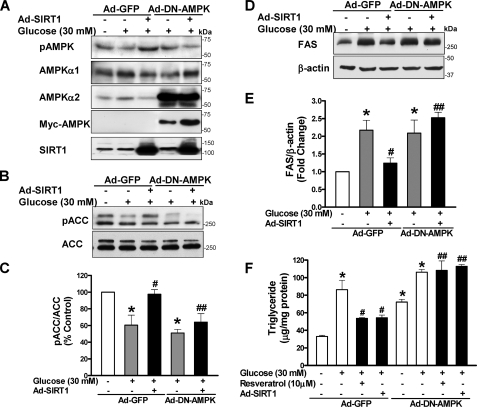
SIRT1 Reduces Hepatocellular Lipid Accumulation through Activation of AMPK—The above data suggest that AMPK may act as an intermediary regulator for the metabolic role of SIRT1. To further examine if SIRT1-dependent AMPK regulates high glucose-induced lipid accumulation, the effect of SIRT1 on AMPK and its downstream effectors, ACC and FAS, was determined in HepG2 cells. As shown in Fig. 6, overexpression of SIRT1 counteracted high glucose-induced suppression of AMPK and ACC phosphorylation, mimicking the effect of polyphenols (Fig. 1B) and constitutively active AMPK (8, 9). In addition, AMPK activation by SIRT1 caused a 45 and 38% decrease in FAS induction and the elevated triglyceride levels induced by high glucose, respectively, which was as effective as resveratrol (Fig. 1). Therefore, similar to the beneficial metabolic effects of polyphenol, SIRT1-dependent AMPK inhibits ACC and FAS, thereby preventing hepatocellular lipid accumulation.
FIGURE 6.
AMPK is required for SIRT1 to suppress FAS induction and lipid accumulation in HepG2 cells exposed to high glucose. HepG2 cells were infected with adenoviral vectors encoding GFP or a Myc-tagged dominant-negative AMPK mutant (Ad-DN-AMPK, AMPKαK45R) or co-infected with Ad-SIRT1 and subsequently incubated for 24 h without or with resveratrol (10 μm) in the absence or presence of high glucose. A, overexpression of the DN-AMPK (∼64 kDa) and SIRT1 (∼120 kDa) was confirmed by immunoblots with anti-Myc and anti-AMPKα2 and with anti-SIRT1 antibodies, respectively. B and C, the ability of SIRT1 to prevent the decrease in ACC phosphorylation caused by high glucose is attenuated by the DN-AMPK. D and E, SIRT1 suppression of high glucose-enhanced FAS expression is abrogated by the DN-AMPK. F, DN-AMPK blocks the effect of SIRT1 and resveratrol on lipid accumulation. *, p < 0.05 versus normal glucose in cells expressing GFP; #, p < 0.05 versus high glucose alone in cells expressing GFP; ##, p < 0.05 versus polyphenol treatment in cells expressing GFP (mean ± S.E., n = 4).
We previously showed that overexpression of a Myc-tagged dominant negative AMPK (DN-AMPK; AMPKαK45R) prevented the increase in AMPK activity and the decrease in lipid accumulation caused by metformin and polyphenols (8, 9). To examine whether AMPK is required for SIRT1 to prevent hepatic lipid accumulation, Ad-DN-AMPK and Ad-SIRT1 were co-infected into HepG2 cells, and ACC phosphorylation, FAS, and lipid levels were determined. Overexpression of DN-AMPK and SIRT1 was confirmed by immunoblots with anti-Myc and anti-SIRT1 antibodies, respectively (Fig. 6A). The DN-AMPK did not alter SIRT1 expression, since neither total cellular nor nuclear SIRT1 was altered (Fig. 6A) (data not shown). Under high glucose conditions, the SIRT1-mediated increase in phosphorylation of AMPK and ACC was largely abrogated by the DN-AMPK (Fig. 6, A–C). As a consequence, the DN-AMPK completely blocked the effect of SIRT1 to suppress FAS induction and triglyceride accumulation as well as the lipid-lowering effect of resveratrol (Fig. 6, D–F), indicating that AMPK is necessary for SIRT1 and resveratrol to lower hepatic lipids. These data demonstrate that AMPK functions as a novel downstream effector for SIRT1 regulation of hepatocyte lipid metabolism.
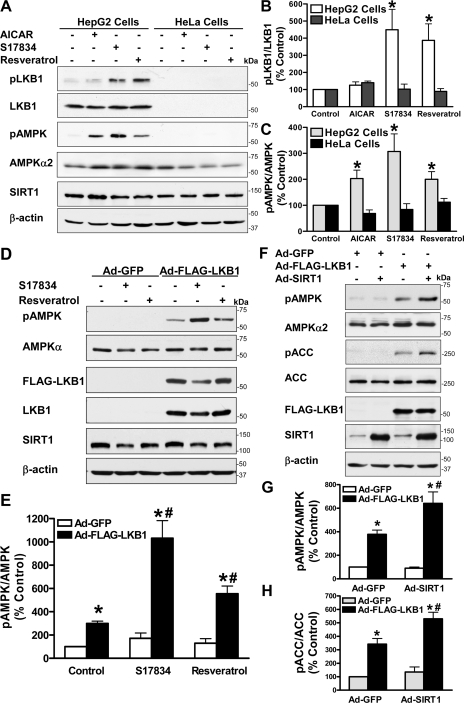
LKB1, but Not CaMKKβ, Mediates the Ability of Polyphenol and SIRT1 to Stimulate AMPK—To date, three AMPK kinases, LKB1, CaMKKβ, and TAK1 (transforming growth factor-β-activated kinase-1), have been identified to phosphorylate and activate AMPK (1–3, 5–7, 41). To elucidate the mechanism underlying the role of SIRT1 in regulating AMPK activity, we further determined the role of LKB1 and CaMKKβ in mediating AMPK activation by polyphenols. Consistent with previous studies showing that CaMKKβ, the predominant isoform of CaMKK present in HeLa cells (5), is responsible for AMPK activation by some stimuli (5, 41), endogenous CaMKKβ, which migrated at the expected apparent molecular mass (∼65 kDa), but not CaMKKα (∼56 kDa), was readily detected by immunoblots with the total CaMKK antibody in HeLa cells (supplemental Fig. 3). As expected, LKB1 was only detectable in HepG2 cells (Fig. 7 and supplemental Fig. 3). Since phosphorylation of LKB1 at Ser428 was shown to regulate LKB1 function (42), the effect of polyphenols on LKB1/AMPK signaling was assessed by determining phosphorylation of both LKB1 at Ser428 and AMPK at Thr172. It has been shown that AICAR, a precursor of the AMP analog ZMP, activates AMPK, but not other LKB1-dependent AMPK-like kinases (43). As shown in Fig. 7, A–C, we indeed found that exposure of HepG2 cells to AICAR (1 mm) increased Thr172 phosphorylation of AMPK by 2-fold but did not alter Ser428 phosphorylation of LKB1. Unlike AICAR, S17834 (10 μm) strongly stimulated phosphorylation of LKB1 by ∼3-fold, as effectively as resveratrol (50 μm), which paralleled increased phosphorylation of AMPK at Thr172, suggesting that polyphenol-activated SIRT1 stimulates LKB1/AMPK signaling in HepG2 cells. In contrast, HeLa cells, which are deficient in LKB1 and represent a natural “knock-out” cell line (5, 44), showed significantly less phosphorylation of both LKB1 and AMPK when treated with polyphenols, implying the importance of LKB1 in polyphenol action on AMPK signaling. We sought to confirm the effect of LKB1 by performing a rescue experiment, in which adenovirus-mediated overexpression of FLAG-tagged wild type LKB1 was infected into LKB1-deficient HeLa cells. Overexpression of LKB1 caused an ∼3-fold increase in the basal phosphorylation of AMPK and ACC and restored polyphenol-induced phosphorylation of AMPK, as compared with their negligible effect in HeLa cells expressing Ad-GFP (Fig. 7, D and E). These results demonstrate that the defect in polyphenol-stimulated LKB1/AMPK signaling in HeLa cells can be attributed to the deficiency in LKB1. We next examined whether LKB1 is also necessary for AMPK activation by SIRT1. Overexpression of SIRT1 was unable to stimulate AMPK in HeLa cells expressing Ad-GFP, and the activation of AMPK by LKB1 was further potentiated by SIRT1 in cells that were reconstituted with wild type LKB1 (Fig. 7, F–H). Collectively, these results indicate that polyphenols and SIRT1 stimulate AMPK signaling via an LKB1-dependent mechanism.
FIGURE 7.
Polyphenols and SIRT1 stimulate AMPK signaling in an LKB1-dependent manner. A–C, phosphorylation of LKB1 at Ser428 and AMPK at Thr172 in response to polyphenols is increased in HepG2 cells expressing LKB1 but not in HeLa cells lacking LKB1. Both human HepG2 hepatocytes and HeLa cells were quiesced in serum-free DMEM overnight and incubated with AICAR (1 mm), S17834 (10 μm), or resveratrol (50 μm) for an additional 1 h. The levels of phosphorylated LKB1 (pLKB1) or AMPK (pAMPK) were normalized to those of total LKB1 or AMPKα2, respectively, and expressed as relative phosphorylation (mean ± S.E., n = 3). *, p < 0.05, versus control in the identical cell lines. D and E, overexpression of LKB1 restores AMPK activation by polyphenols in LKB1-deficient HeLa cells. Adenovirus-mediated vectors encoding either GFP (Ad-GFP) or FLAG-tagged wild type LKB1 (Ad-FLAG-LKB1) were infected into HeLa cells, followed by treatment with S17834 (10 μm) or resveratrol (50 μm) for 1 h as indicated. F–H, SIRT1-dependent AMPK signaling is restored by overexpression of LKB1 in HeLa cells. Ad-GFP, Ad-FLAG-LKB1, or Ad-SIRT1 was infected into HeLa cells as indicated. Immunoblots with anti-FLAG, anti-LKB1, or anti-SIRT1 antibodies confirmed overexpression of recombinant FLAG-LKB1 (∼60 kDa) or SIRT1 (∼120 kDa). *, p < 0.05 versus Ad-GFP alone; #, p < 0.05 versus Ad-FLAG-LKB1 alone (mean ± S.E., n = 3).
To further assess whether AMPK activation by polyphenols is mediated by an alternate upstream kinase, CaMKKβ, STO-609, a relatively selective and cell permeable inhibitor of CaMKK (3, 5, 41, 45), was used to distinguish the effects mediated by CaMKKβ or by LKB1 in both human cell lines. As shown in supplemental Fig. 3, A and B, inhibition of CaMKKβ by STO-609 totally ablated the basal phosphorylation of AMPK and ACC in HeLa cells that lack LKB1 but express CaMKKβ. By contrast, LKB1-dependent AMPK activity in the absence or presence of resveratrol was unaffected by STO-609 in HeLa cells that were reconstituted with wild type LKB1, suggesting that CaMKKβ is only responsible for the basal but not polyphenol-stimulated AMPK in HeLa cells. Moreover, in HepG2 cells that may express LKB1, CAMKKα, and CAMKKβ, pharmacological inhibition of CaMKK by STO-609 also had no inhibitory effect on the phosphorylation of LKB1, AMPK, and ACC that was stimulated by S17834 in HepG2 cells, as was seen in HeLa cells (supplemental Fig. 3, C–E). Although the basal phosphorylation of AMPK and ACC was unchanged by STO-609 in HepG2 cells, this might suggest that LKB1 or other mechanisms maintain the basal AMPK activity in hepatocytes. Taken together, our results indicate that LKB1 acts as the major AMPK kinase to mediate the role of polyphenols and SIRT1 on AMPK signaling.
DISCUSSION
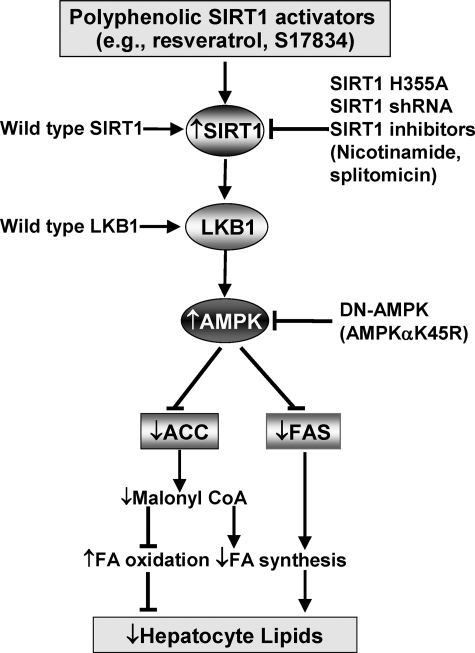
The present studies provide the first direct evidence that SIRT1 stimulates AMPK activation via an LKB1-dependent manner and that SIRT1-mediated AMPK activation represents a novel mechanism of the beneficial effects of polyphenols on hepatocyte lipid metabolism. We showed here that polyphenols, including resveratrol and the synthetic polyphenol, S17834, increased NAD+-dependent deacetylase activity of SIRT1 as well as enhanced phosphorylation of AMPK at Thr172 and its upstream kinase LKB1 at Ser428, leading to increased phosphorylation of ACC. Overexpression of wild type SIRT1 also enhanced the basal phosphorylation of AMPK and ACC in hepatocytes and in the mouse liver in vivo as well as countered elevated expression of FAS and accumulated lipids caused by high glucose, which has been linked to hyperlipidemia in diabetes (9). The lipid-lowering effect of SIRT1 was completely abolished by DN-AMPK. In addition, the ability of polyphenols to stimulate AMPK and reduce hepatocellular lipid accumulation was mimicked by overexpression of SIRT1 and abolished by knockdown or pharmacological inhibition of SIRT1. Furthermore, in LKB-deficient HeLa cells, polyphenols and SIRT1 were unable to stimulate AMPK activation, and the activation was reconstituted by overexpression of LKB1. Also, the activation of AMPK caused by polyphenols is not affected by pharmacological inhibition of CaMKK. Although other factors may contribute to the hepatocyte metabolic effects of SIRT1, the present study provides an attractive model in which SIRT1 activation by polyphenols stimulates AMPK via an LKB1-dependent manner. SIRT1-dependent AMPK activation functionally inhibits ACC and FAS that are key downstream regulators of AMPK in the control of lipid metabolism and consequently reduces lipid accumulation, possibly through increased fatty acid oxidation and/or decreased fatty acid synthesis (Fig. 8).
FIGURE 8.
Proposed scheme for the role of SIRT1-activating polyphenols in the regulation of AMPK signaling and hepatocyte lipid metabolism. SIRT1-activating polyphenols, such as resveratrol and S17834, stimulate LKB1 phosphorylation as well as AMPK phosphorylation and activation. Similarly, overexpression of wild type SIRT1 also increases AMPK phosphorylation, which in turn increases ACC phosphorylation and inhibits ACC activity. As a consequence, decreased production of malonyl-CoA results in up-regulation of fatty acid oxidation as well as down-regulation of fatty acid synthesis, thereby leading to hepatocyte lipid reduction. On the other hand, activation of AMPK by polyphenols inhibits glucose-induced expression of FAS, which contributes to the reduction in triglycerides through inhibition of fatty acid biosynthesis. Moreover, SIRT1 is required for the effects of polyphenols on AMPK and lipids, since the beneficial effects of polyphenols are mimicked by overexpression of wild type SIRT1 and abrogated by inhibition of SIRT1, such as the inactive SIRT1 mutant (SIRT1H355A), shRNA SIRT1, or inhibitors of SIRT1. Importantly, the stimulation of AMPK and the lipid-lowering effect of SIRT1 are abolished by DN-AMPK, suggesting that AMPK acts as a novel functional downstream regulator for the metabolic effect of SIRT1. In addition, polyphenols and SIRT1 stimulate AMPK via an LKB1-dependent mechanism. Therefore, SIRT1/LKB1/AMPK signaling may be considered a novel molecular mechanism for potential therapeutic effects of polyphenols on hepatic lipid accumulation in diabetes and age-related metabolic disorders.
SIRT1 Positively Regulates AMPK Signaling—The most important implication of the present studies is that SIRT1 plays a critical role in stimulating AMPK signaling in hepatocytes and other cell types. Several lines of molecular and biochemical evidence have previously supported functional connections between the two master metabolic regulators, SIRT1 and AMPK. The life span of Caenorhabditis elegans is increased by overexpression of sir2.1 or aak-2 (AMPK) and blocked by loss of sir2.1 or aak-2 (46, 47). SIRT1 has been implicated in the regulation of aging and age-related diseases (48). Aging-associated reduction in AMPK activity also contributes to dysregulated lipid metabolism (22). Both SIRT1 and AMPK play a critical role in glucose and lipid metabolism (4, 9, 18, 23, 34). In the present study, the dose-dependent effect of resveratrol on SIRT1 activity closely correlated with that of AMPK stimulation. S17834, a synthetic polyphenol that we previously showed increased AMPK phosphorylation in the liver of diabetic mice, was also demonstrated to increase SIRT1-dependent AMPK activity. These findings are consistent with the previous observation that SIRT1 deacetylation activity and AMPK activity are increased in the liver of mice treated with resveratrol (12). Furthermore, our finding that knockdown of SIRT1 by shRNA decreased the basal AMPK activity suggests that endogenous SIRT1 activity contributes to the maintenance of the AMPK in an active state. In contrast, overexpression of SIRT1 stimulated hepatic AMPK and ACC phosphorylation in vitro and in vivo. Thus, genetic manipulation of SIRT1 modulates hepatic AMPK activity and its downstream signaling, suggesting that SIRT1 functions as a novel upstream regulator of AMPK signaling.
SIRT1-dependent AMPK Activation Regulates Hepatocyte Lipid Metabolism—Because overexpression of SIRT1 decreases lipid accumulation in adipocytes (34) and hepatocytes (18), it raises the possibility that SIRT1 functions as a critical regulator of AMPK in the control of lipid metabolism. AMPK was originally identified through its phosphorylation and inactivation of ACC in the liver (49, 50). AMPK-mediated ACC inhibition, through reducing malonyl-CoA biosynthesis, plays a key role in fatty acid oxidation and synthesis (51), because liver-specific deletion of ACC1 reduces hepatic triglyceride accumulation by decreasing de novo fatty acid synthesis (33), and ACC2 knock-out mice display a higher rate of fatty acid oxidation (52). In the present study, the lipid-lowering effect of SIRT1 can be attributed to its ability to regulate the downstream effectors of AMPK. For instance, SIRT1-mediated activation of AMPK increases ACC phosphorylation and therefore inhibits its effects on fatty acid synthesis and oxidation. In addition to ACC, inhibition of FAS, a target lipogenic enzyme for the transcription factor SREBP, is another important consequence of AMPK activation by polyphenols and SIRT1. Constitutively active AMPK and AICAR both inhibit glucose-activated expression of the FAS gene in hepatocytes (53). These findings define a novel molecular mechanism by which SIRT1 regulates hepatic lipid metabolism through activation of AMPK and suppression of its downstream effectors, ACC and FAS. Therefore, like the effect of AMPK activation by metformin (8, 10), the lipid-lowering effect of SIRT1 can be explained by increased fatty acid oxidation and decreased fatty acid synthesis. This conclusion is also strengthened by the evidence that hepatic overexpression of SIRT1 in vivo reduces lipids by down-regulation of lipogenic gene expression (e.g. SREBP-1 and FAS) and up-regulation of expression of genes that control fatty acid oxidation (18).
Another major finding of the present study is that the effect of SIRT1 overexpression on ACC phosphorylation and FAS expression as well as lipid accumulation was completely abrogated by the DN-AMPK, indicating that AMPK is a key downstream regulator of the lipid metabolic effects of SIRT1. The importance of AMPK in the process is also evidenced by the fact that the decrease in FAS expression and/or improvement in lipid accumulation and fatty liver in resveratrol- or S17834-treated diabetic mice (9, 12) may be attributed to increased SIRT1 and AMPK activity. In addition, although we found that nicotinamide or an SIRT1 inactive mutant had little effect on basal lipid levels in HepG2 cells, it is possible that partial inhibition of SIRT1 is not sufficient to affect basal AMPK activity and its metabolic functions. Therefore, our studies support a model for the role of SIRT1-dependent AMPK activation in the regulation of hepatocyte lipid accumulation (Fig. 8). These underlying mechanisms may explain the therapeutic effects of polyphenols to reduce hyperlipidemia and vascular complications in diabetes in vivo (9, 12, 20).
SIRT1 Stimulates AMPK Signaling in an LKB1-dependent Mechanism—Previous studies indicated that polyphenols stimulate AMPK via an indirect mechanism (12), suggesting that the upstream kinases of AMPK, LKB1, and CaMKKβ are involved in the effect of SIRT1 on AMPK. The present study demonstrating that phosphorylation of AMPK and ACC was not increased in response to polyphenols or SIRT1 overexpression in LKB1-deficient HeLa cells, and that the increase is restored by overexpression of wild type LKB1, strongly supports the notion that AMPK activation by SIRT1 and polyphenols is mediated by LKB1. These findings are consistent with the recent study that resveratrol-stimulated AMPK activity is abolished in LKB1-deficient neurons (11). Furthermore, because of the lack of inhibitory effect of STO-609, the effect of polyphenols on AMPK was independent of CaMKKβ.
Because the mice with liver-specific knock-out of LKB1 display a complete loss of AMPK activity and increased gene/protein expression of lipogenic enzymes and block the ability of metformin to activate AMPK and lower glucose (2), LKB1 appears to be an attractive candidate as an intermediate of SIRT1/AMPK signaling in regulating hepatic lipid homeostasis. Phosphorylation of LKB1 at Ser428 and AMPK at Thr172 was coincidentally stimulated by polyphenols in HepG2 cells. Importantly, LKB1 Ser428 was previously shown to be phosphorylated by other protein kinases, such as PKCζ, PKA, and p90RSK (42, 54). Mutation of LKB1 Ser428 to Ala abolishes the effect of metformin on AMPK, like the dominant negative mutant of LKB1 (54). Although the precise mechanism by which SIRT1 activation by polyphenols leads to LKB1-dependent AMPK activation remains unclear, it is possible that SIRT1 regulates LKB1 via a mechanism involving its direct deacetylation or Ser428 phosphorylation of LKB1, which might be mediated by other protein kinases. Whether such a mechanism has a role in the regulation of lipid metabolism controlled by SIRT1 is worthy of additional investigation.
Polyphenols Stimulate AMPK and Prevent Hepatocyte Lipid Accumulation by Activating SIRT1—The present studies strongly suggest that the activation by polyphenols of AMPK and their lipid metabolic effects are at least partially dependent on SIRT1, since these effects of polyphenols were mimicked by increased expression of SIRT1 and diminished by knockdown or inhibition of SIRT1. These results are consistent with SIRT1 being the molecular target of polyphenols (24) and the main mediator of their protection against metabolic disease in vivo (12, 20, 21). In contrast, resveratrol-stimulated AMPK activation in neurons was shown to be independent of SIRT1 (11). Given the accumulating evidence that different isoforms of AMPK subunits and the upstream kinases of AMPK display tissue-specific effects (23) and that metformin activates AMPK in the liver (8, 10) but inhibits AMPK in neurons (55), the most likely explanation is that SIRT1 regulation of AMPK signaling is also tissue-specific. Since SIRT1 interacts with and deacetylates other substrates, such as PGC-1α, FOXO1, and PPARγ (18, 34, 56), it is possible that such regulators are involved in the metabolic effects of SIRT1/AMPK signaling in hepatocytes. Importantly, PGC-1α is also directly phosphorylated by AMPK (57). Because the current study has demonstrated that AMPK is required for SIRT1 to inhibit lipid accumulation, it is conceivable that SIRT1 regulates hepatic lipid homeostasis possible through AMPK-mediated phosphorylation of PGC-1α.
In conclusion, the present study establishes a molecular cross-talk between SIRT1- and LKB1/AMPK signaling in the regulation of hepatocyte lipid metabolism. Our findings have potential implications in polyphenols protecting against hepatocellular lipid accumulation and acceleration of atherosclerosis associated with diabetes and age-related metabolic disorders.
Supplementary Material
Acknowledgments
We greatly thank Dr. Junichi Sadoshima (New Jersey Medical School) for the adenoviral vector encoding the wild type SIRT1 and Drs. Pere Puigserver and Joseph T. Rodgers (Harvard Medical School) for the adenoviral vectors encoding FLAG-tagged wild type and H355A mutant of SIRT1 as well as control shRNA and SIRT1 shRNA. We appreciate the important discussion with and helpful advice from Dr. Neil Ruderman. We also acknowledge Dr. Yasuhiro Izumiya for technical assistance in mouse adenovirus injection.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 HL 68758, R01 DK76942, and R01 AG27080. This work was supported in part by a Strategic Alliance between the Vascular Biology Unit at Boston University Medical Center and Institut de Recherches Servier. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
Footnotes
The abbreviations used are: AMPK, AMP-activated protein kinase; DN-AMPK, dominant negative AMPK; ACC, acetyl-CoA carboxylase; AICAR, 5-amino-4-imidazolecarboxamide riboside; CaMKKβ, Ca2+/calmodulin-dependent protein kinase kinase β; S17834, a synthetic polyphenol (6,8-diallyl-5,7-dihydroxy-2-(2-allyl-3-hydroxy-4-methoxyphenyl)1-H benzo-(b)pyran-4-one); shRNA, short hairpin RNA; GFP, green fluorescent protein; DMEM, Dulbecco's modified Eagle's medium; Ad, adenovirus.
References
- 1.Shaw, R. J., Kosmatka, M., Bardeesy, N., Hurley, R. L., Witters, L. A., DePinho, R. A., and Cantley, L. C. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw, R. J., Lamia, K. A., Vasquez, D., Koo, S. H., Bardeesy, N., DePinho, R. A., Montminy, M., and Cantley, L. C. (2005) Science 310 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurley, R. L., Anderson, K. A., Franzone, J. M., Kemp, B. E., Means, A. R., and Witters, L. A. (2005) J. Biol. Chem. 280 29060–29066 [DOI] [PubMed] [Google Scholar]
- 4.Long, Y. C., and Zierath, J. R. (2006) J. Clin. Invest. 116 1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawley, S. A., Pan, D. A., Mustard, K. J., Ross, L., Bain, J., Edelman, A. M., Frenguelli, B. G., and Hardie, D. G. (2005) Cell Metab. 2 9–19 [DOI] [PubMed] [Google Scholar]
- 6.Momcilovic, M., Hong, S. P., and Carlson, M. (2006) J. Biol. Chem. 281 25336–25343 [DOI] [PubMed] [Google Scholar]
- 7.Xie, M., Zhang, D., Dyck, J. R. B., Li, Y., Zhang, H., Morishima, M., Mann, D. L., Taffet, G. E., Baldini, A., Khoury, D. S., and Schneider, M. D. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 17378–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zang, M. W., Zuccollo, A., Hou, X. Y., Nagata, D., Walsh, K., Herscovitz, H., Brecher, P., Ruderman, N. B., and Cohen, R. A. (2004) J. Biol. Chem. 279 47898–47905 [DOI] [PubMed] [Google Scholar]
- 9.Zang, M. W., Xu, S. Q., Maitland-Toolan, K. A., Zuccollo, A., Hou, X. Y., Jiang, B. B., Wierzbicki, M., Verbeuren, T. J., and Cohen, R. A. (2006) Diabetes 55 2180–2191 [DOI] [PubMed] [Google Scholar]
- 10.Zhou, G. C., Myers, R., Li, Y., Chen, Y. L., Shen, X. L., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber, T., Fujii, N., Musi, N., Hirshman, M. F., Goodyear, L. J., and Moller, D. E. (2001) J. Clin. Invest. 108 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta, B., and Milbrandt, J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baur, J. A., Pearson, K. J., Price, N. L., Jamieson, H. A., Lerin, C., Kalra, A., Prabhu, V. V., Allard, J. S., Lopez-Lluch, G., Lewis, K., Pistell, P. J., Poosala, S., Becker, K. G., Boss, O., Gwinn, D., Wang, M. Y., Ramaswamy, S., Fishbein, K. W., Spencer, R. G., Lakatta, E. G., Le Couteur, D., Shaw, R. J., Navas, P., Puigserver, P., Ingram, D. K., de Cabo, R., and Sinclair, D. A. (2006) Nature 444 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blander, G., and Guarente, L. (2004) Annu. Rev. Biochem. 73 417–435 [DOI] [PubMed] [Google Scholar]
- 14.Leibiger, I. B., and Berggren, P. O. (2006) Nat. Med. 12 34–36 [DOI] [PubMed] [Google Scholar]
- 15.Gerhart-Hines, Z., Rodgers, J. T., Bare, O., Kim, C. L. S. H., Kim, S. H., Mostoslavsky, R., Alt, F. W., Wu, Z. D., and Puigserver, P. (2007) EMBO J. 26 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarente, L. (2006) Nature 444 868–874 [DOI] [PubMed] [Google Scholar]
- 17.Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., and Puigserver, P. (2005) Nature 434 113–118 [DOI] [PubMed] [Google Scholar]
- 18.Rodgers, J. T., and Puigserver, P. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 12861–12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, C., Zhang, F., Ge, X. J., Yan, T. T., Chen, X. M., Shi, X. L., and Zhai, Q. W. (2007) Cell Metab. 6 307–319 [DOI] [PubMed] [Google Scholar]
- 20.Lagouge, M., Argmann, C., Gerhart-Hines, Z., Meziane, H., Lerin, C., Daussin, F., Messadeq, N., Milne, J., Lambert, P., Elliott, P., Geny, B., Laakso, M., Puigserver, P., and Auwerx, J. (2006) Cell 127 1109–1122 [DOI] [PubMed] [Google Scholar]
- 21.Milne, J. C., Lambert, P. D., Schenk, S., Carney, D. P., Smith, J. J., Gagne, D. J., Jin, L., Boss, O., Perni, R. B., Vu, C. B., Bemis, J. E., Xie, R., Disch, J. S., Ng, P. Y., Nunes, J. J., Lynch, A. V., Yang, H. Y., Galonek, H., Israelian, K., Choy, W., Iffland, A., Lavu, S., Medvedik, O., Sinclair, D. A., Olefsky, J. M., Jirousek, M. R., Elliott, P. J., and Westphal, C. H. (2007) Nature 450 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reznick, R. M., Zong, H. H., Li, J., Morino, K., Moore, I. K., Yu, H. J., Liu, Z. X., Dong, J. Y., Mustard, K. J., Hawley, S. A., Befroy, D., Pypaert, M., Hardie, D. G., Young, L. H., and Shulman, G. I. (2007) Cell Metab. 5 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn, B. B., Alquier, T., Carling, D., and Hardie, D. G. (2005) Cell Metab. 1 15–25 [DOI] [PubMed] [Google Scholar]
- 24.Howitz, K. T., Bitterman, K. J., Cohen, H. Y., Lamming, D. W., Lavu, S., Wood, J. G., Zipkin, R. E., Chung, P., Kisielewski, A., Zhang, L. L., Scherer, B., and Sinclair, D. A. (2003) Nature 425 191–196 [DOI] [PubMed] [Google Scholar]
- 25.Zang, M. W., Waelde, C. A., Xiang, X. Q., Rana, A., Wen, R., and Luo, Z. J. (2001) J. Biol. Chem. 276 25157–25165 [DOI] [PubMed] [Google Scholar]
- 26.Cianflone, K., Dahan, S., Monge, J. C., and Sniderman, A. D. (1992) Arterioscler. Thromb. 12 271–277 [DOI] [PubMed] [Google Scholar]
- 27.Nakajima, K., Yamauchi, K., Shigematsu, S., Ikeo, S., Komatsu, M., Aizawa, T., and Hashizume, K. (2000) J. Biol. Chem. 275 20880–20886 [DOI] [PubMed] [Google Scholar]
- 28.Alcendor, R. R., Kirshenbaum, L. A., Imai, S., Vatner, S. F., and Sadoshima, J. (2004) Circ. Res. 95 971–980 [DOI] [PubMed] [Google Scholar]
- 29.Zang, M. W., Dong, M. Q., Pinon, D. I., Ding, X. Q., Hadac, E. M., Li, Z. J., Lybrand, T. P., and Miller, L. J. (2003) Mol. Pharmacol. 63 993–1001 [DOI] [PubMed] [Google Scholar]
- 30.Liao, W., Nguyen, M. T. A., Imamura, T., Singer, O., Verma, I. M., and Olefsky, J. M. (2006) Endocrinology 147 2245–2252 [DOI] [PubMed] [Google Scholar]
- 31.Zang, M., Hayne, C., and Luo, Z. (2002) J. Biol. Chem. 277 4395–4405 [DOI] [PubMed] [Google Scholar]
- 32.Lee, Y., Yu, X., Gonzales, F., Mangelsdorf, D. J., Wang, M. Y., Richardson, C., Witters, L. A., and Unger, R. H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 11848–11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao, J., Demayo, F. J., Li, H., Abu-Elheiga, L., Gu, Z., Shaikenov, T. E., Kordari, P., Chirala, S. S., Heird, W. C., and Wakil, S. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 8552–8557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado, D. O., Leid, M., McBurney, M. W., and Guarente, L. (2004) Nature 429 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, Y. X., Lee, C. H., Tiep, S., Yu, R. T., Ham, J., Kang, H., and Evans, R. M. (2003) Cell 113 159–170 [DOI] [PubMed] [Google Scholar]
- 36.Shibata, R., Ouchi, N., Ito, M., Kihara, S., Shiojima, I., Pimentel, D. R., Kumada, M., Sato, K., Schiekofer, S., Ohashi, K., Funahashi, T., Colucci, W. S., and Walsh, K. (2004) Nat. Med. 10 1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postic, C., Dentin, R., and Girard, J. (2004) Diabetes Metab. 30 398–408 [DOI] [PubMed] [Google Scholar]
- 38.Hirao, M., Posakony, J., Nelson, M., Hruby, H., Jung, M. F., Simon, J. A., and Bedalov, A. (2003) J. Biol. Chem. 278 52773–52782 [DOI] [PubMed] [Google Scholar]
- 39.Luo, J., Nikolaev, A. Y., Imai, S., Chen, D., Su, F., Shiloh, A., Guarente, L., and Gu, W. (2001) Cell 107 137–148 [DOI] [PubMed] [Google Scholar]
- 40.Vaziri, H., Dessain, S. K., Eagon, E. N., Imai, S. I., Frye, R. A., Pandita, T. K., Guarente, L., and Weinberg, R. A. (2001) Cell 107 149–159 [DOI] [PubMed] [Google Scholar]
- 41.Woods, A., Dickerson, K., Heath, R., Hong, S. P., Momcilovic, M., Johnstone, S. R., Carlson, M., and Carling, D. (2005) Cell Metab. 2 21–33 [DOI] [PubMed] [Google Scholar]
- 42.Alessi, D. R., Sakamoto, K., and Bayascas, J. R. (2006) Annu. Rev. Biochem. 75 137–163 [DOI] [PubMed] [Google Scholar]
- 43.Lizcano, J. M., Goransson, O., Toth, R., Deak, M., Morrice, N. A., Boudeau, J., Hawley, S. A., Udd, L., Makela, T. P., Hardie, D. G., and Alessi, D. R. (2004) EMBO J. 23 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawley, S. A., Boudeau, J., Reid, J. L., Mustard, K. J., Udd, L., Makela, T. P., Alessi, D. R., and Hardie, D. G. (2003) J. Biol. 2 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tokumitsu, H., Inuzuka, H., Ishikawa, Y., Ikeda, M., Saji, I., and Kobayashi, R. (2002) J. Biol. Chem. 277 15813–15818 [DOI] [PubMed] [Google Scholar]
- 46.Curtis, R., O'Connor, G., and DiStefano, P. S. (2006) Aging Cell 5 119–126 [DOI] [PubMed] [Google Scholar]
- 47.Narbonne, P., and Roy, R. (2006) Development 133 611–619 [DOI] [PubMed] [Google Scholar]
- 48.Haigis, M. C., and Guarente, L. P. (2006) Genes Dev. 20 2913–2921 [DOI] [PubMed] [Google Scholar]
- 49.Hardie, D. G. (2004) Rev. Endocr. Metab. Disord. 5 119–125 [DOI] [PubMed] [Google Scholar]
- 50.Park, S. H., Gammon, S. R., Knippers, J. D., Paulsen, S. R., Rubink, D. S., and Winder, W. W. (2002) J. Appl. Physiol. 92 2475–2482 [DOI] [PubMed] [Google Scholar]
- 51.Ruderman, N., and Prentki, M. (2004) Nat. Rev. Drug Discov. 3 340–351 [DOI] [PubMed] [Google Scholar]
- 52.Abu-Elheiga, L., Matzuk, M. M., Abo-Hashema, K. A., and Wakil, S. J. (2001) Science 291 2613–2616 [DOI] [PubMed] [Google Scholar]
- 53.Woods, A., Azzout-Marniche, D., Foretz, M., Stein, S. C., Lemarchand, P., Ferre, P., Foufelle, F., and Carling, D. (2000) Mol. Cell. Biol. 20 6704–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie, Z., Dong, Y., Scholz, R., Neumann, D., and Zou, M. H. (2008) Circulation 117 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chau-Van, C., Gamba, M., Salvi, R., Gaillard, R. C., and Pralong, F. P. (2007) Endocrinology 148 507–511 [DOI] [PubMed] [Google Scholar]
- 56.Daitoku, H., Hatta, M., Matsuzaki, H., Aratani, S., Ohshima, T., Miyagishi, M., Nakajima, T., and Fukamizu, A. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jager, S., Handschin, C., St Pierre, J., and Spiegelman, B. M. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.