Abstract
The R403Q mutation in the β-myosin heavy chain (MHC) was the first mutation to be linked to familial hypertrophic cardiomyopathy (FHC), a primary disease of heart muscle. The initial studies with R403Q myosin, isolated from biopsies of patients, showed a large decrease in myosin motor function, leading to the hypothesis that hypertrophy was a compensatory response. The introduction of the mouse model for FHC (the mouse expresses predominantly α-MHC as opposed to the β-isoform in larger mammals) created a new paradigm for FHC based on finding enhanced motor function for R403Q α-MHC. To help resolve these conflicting mechanisms, we used a transgenic mouse model in which the endogenous α-MHC was largely replaced with transgenically encoded β-MHC. A His6 tag was cloned at the N terminus of the α-and β-MHC to facilitate protein isolation by Ni2+-chelating chromatography. Characterization of the R403Q α-MHC by the in vitro motility assay showed a 30-40% increase in actin filament velocity compared with wild type, consistent with published studies. In contrast, the R403Q mutation in a β-MHC backbone showed no enhancement in velocity. Cleavage of the His-tagged myosin by chymotrypsin made it possible to isolate homogeneous myosin subfragment 1 (S1), uncontaminated by endogenous myosin. We find that the actin-activated MgATPase activity for R403Q α-S1 is ∼30% higher than for wild type, whereas the enzymatic activity for R403Q β-S1 is reduced by ∼10%. Thus, the functional consequences of the mutation are fundamentally changed depending upon the context of the cardiac MHC isoform.
The arginine to glutamine mutation at amino acid 403 (R403Q) in the β-myosin heavy chain (MHC)2 was the first MHC missense mutation linked to familial hypertrophic cardiomyopathy (FHC), a primary disease of heart muscle (1). Since that time several hundred mutations in genes encoding sarcomeric proteins have been identified, leading to the designation of FHC as primarily a “disease of the sarcomere” (2, 3). However, the pathways linking the various genetic defects to the characteristic human disease phenotype remain largely unknown. Even the fundamental question of whether these mutations cause a gain or a loss of function in myosin remains unresolved. We have confined our study to the R403Q mutation, because it is one of the most extensively studied FHC mutations and because it has a poor clinical prognosis (2, 4). The majority of the earlier biochemical/biophysical results, derived from expression systems and biopsies, supported the hypothesis that the R403Q mutation leads to a large decrease in motor function, which ultimately results in a compensatory hypertrophic response (for review, see Ref. 5).
The first murine model for FHC replaced one allele of the endogenous cardiac mouse α-MHC with the mutant α-MHC (R403Q) gene by homologous recombination (6); this heterozygous mouse (R403Q/+) resembled the human cardiac phenotype in many ways, except cardiac hypertrophy was notably milder. Subsequent studies on myosin extracted from a <1-week-old, homozygous mouse (R403Q/R403Q) (homozygotes do not survive longer than a week) gave the unexpected finding of a large gain in function by motility and ATPase assays (7). A limitation of the mouse model is the presence of predominantly α-MHC in the postnatal mouse ventricle. All larger mammals, including humans, have the slower β-cardiac myosin expressed predominantly in their ventricles, and the 2-fold faster α-cardiac myosin is confined to the atria. Because the α- and β-myosin isoforms of all species are 93% identical in amino acid sequence, it has been generally assumed that the different backbones would have few, if any, consequences for function. Here we test this assumption by comparing the functional properties of R403Q expressed in β-cardiac MHC to the same mutation expressed in α-cardiac MHC in a transgenic mouse model.
This study was made possible through the use of transgenesis to replace the endogenous α-cardiac myosin with expressed β-cardiac myosin (8). Even with as much as 70% wild-type β-MHC, no detrimental effects on cardiac morphology were observed other than the expected decrease in contractile function, and the mice had a normal life span (8). To facilitate the isolation of mouse α- and β-cardiac myosin containing the R403Q mutation, a His6 tag was cloned onto the N terminus of α-MHC and β-MHC along with the mutation. Purified myosin containing a minimum of one His tag/molecule (heterodimers of His-MHC/endogenous MHC) could be readily isolated by Ni2+-chelating affinity chromatography. To obtain a homogeneous population of mutant myosin heads, crude myosin was first digested with α-chymotrypsin to yield subfragment-1 (S1). The His-tagged S1(R403Q) could then be isolated by Ni2+ chromatography. Characterization of the mutant myosin and S1 by the in vitro motility assay and actin-activated MgATPase activity, respectively, indicated that the functional effects of R403Q depend on the isoform type carrying the mutation. Whereas the R403Q mutation in an α-backbone, the predominant isoform in small rodents, showed an increase in motility and enzymatic activity relative to wild type, the same mutation in a β-backbone had little observable effect on unloaded motility and a slight loss of enzymatic activity relative to wild-type β-myosin. We conclude that the mechanistic effect of an FHC mutation is best determined in a β-MHC backbone if the ultimate goal is to relate the findings to cardiomyopathy in humans.
EXPERIMENTAL PROCEDURES
Generation of Transgenic Mice—All animal procedures were in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital and the University of Vermont College of Medicine. Generation and phenotype analysis of transgenic (TG) mice with cardiac-specific expression of the parental α-MHC and β-MHC and the generation of the promoter and full-length cDNA constructs have been described previously (8). The His tag was added using the overlapping PCR method with flanking primers. All PCR products were sequenced completely, and the fragments were linked to the mouse α-MHC promoter (Fig. 1). The final constructs were digested free of vector sequence with NotI, purified from agarose gels, and used to generate TG mice. Mutant and wild-type His-tagged α-MHC mice were generated at the University of Vermont Mouse Facility, whereas the TG β-MHC mice were prepared at Cincinnati Children's Hospital. To enhance the degree of replacement of the endogenous α-MHC with the mutant β-MHC, the TG line was bred into the heterogeneous α-MHC null background (9). To shift the isoform expression in non-transgenic hearts from α- to β-MHC, adult mice were treated with propylthiouracil (PTU) for 5-11 weeks (10).
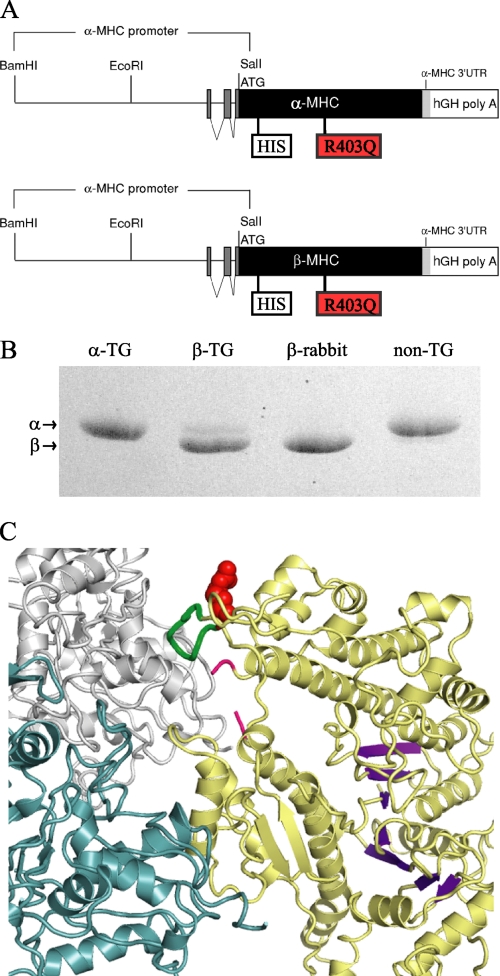
FIGURE 1.
TG expression of α- and β-MHC in mouse hearts. A, diagrams of the TG constructs (adapted from Krenz et al. (8)) modified to contain a His tag at the N terminus and the point mutation R403Q. hGH, human growth hormone. B, expressed mouse wild-type proteins (α-TG and β-TG) and myosin isolated from non-transgenic rabbit and mouse ventricles on 5% glycerol, SDS separation gels. It is evident that the α- and β-MHC isoforms are well separated in this gel system. C, atomic model of smooth muscle myosin docked on actin based on electron cryomicroscopy reconstructions (17). Arginine 403 is shown in red spheres at the base of the cardiomyopathy loop, colored green. Two actin monomers at the interface are shown in gray and blue. The motor domain is colored yellow, and the visible ends of loop 2 are marked in red. The remainder of loop 2 is not seen in the crystal structure used for the docking (47). The β-sheet of seven strands at the active site is shown in magenta.
Protein Purification—Cardiac myosin was prepared from ∼1 g of tissue (10 or more frozen mouse hearts). The thawed tissue was homogenized in 40 mm imidazole at pH 7.2, 2 mm MgCl2, and 1 μg/ml leupeptin (Sorvall Omni-Mixer) and clarified in the same buffer by centrifugation (Sorvall RC5C Plus) until the supernatant was colorless. The pellet was homogenized in 15 ml of extraction buffer (150 mm sodium phosphate, pH 7.0, 0.3 m NaCl, 10 mm sodium pyrophosphate, 2 mm MgCl2, 1 mm EGTA, 1 mm DTT, 1 μg/ml leupeptin, 0.5 mm 1-chloro-3-to-sylamido-7-amino-2-heptanone, 0.5 mm 4-(2-aminoethyl)benzenesulfonyl fluoride), and the suspension was stirred for 30 min. After centrifuging the suspension (Beckman Ti 70 rotor for 45 min at 40,000 rpm), the supernatant was diluted 12-fold with water containing 0.5 mm DTT. The precipitated protein was collected after ∼45 min by centrifugation (Sorvall). The pellets were dissolved in 0.5 m NaCl, 25 mm sodium phosphate, pH 7.0, 1 mm EGTA, 0.2 mm DTT, 1 μg/ml leupeptin, and dialyzed overnight in the cold against the same buffer. This preparation was used as the starting material for all subsequent procedures.
Myosin, which did not have a His tag, was further purified by hydrophobic interaction chromatography on a prepacked 5-ml Toyopearl MD-G Ether-650S column (10 mm × 6.8 cm, Tosoh Biosciences) as described previously (11). An equal volume of a 2× stock of buffer A (1.4 m ammonium sulfate, 20 mm imidazole, pH 6.8, 2 mm MgCl2, 0.2 mm EDTA, and 1 mm NaN3) was added to the dialyzed myosin preparation, and DTT and MgATP were added to a concentration of 0.5 mm each. The myosin was centrifuged in the Beckman Optima MAX-E Ultracentrifuge in the MLA-80 rotor at 78,000 rpm for 30 min at room temperature and loaded onto the column equilibrated in buffer A, DTT, and MgATP. Using an AKTA fast performance liquid chromatography system, the protein was eluted by reducing the concentration of ammonium sulfate in a step from 1.4 to 1.2 m with buffer B, which was identical to buffer A except for the absence of ammonium sulfate. The protein was collected in 2-3 1-ml fractions and dialyzed at least 4 h versus 0.5 m NaCl, 25 mm imidazole, pH 7.0, and 1 mm DTT to reduce the ammonium sulfate concentration. The protein was concentrated ∼4-fold by dialysis overnight against 55% glycerol buffer containing 0.4 m KCl, 10 mm imidazole, pH 7.5, 1 mm EGTA, 1 mm MgCl2, 1 mm sodium azide, 1 mm DTT, and stored at -20 °C.
Myosin with a His tag was isolated by using nickel-chelating affinity chromatography. After clarification, the myosin preparation was loaded onto a 5-ml HiTrap-chelating HP column (Amersham Biosciences). Buffer A consisting of 0.5 m NaCl and 20 mm HEPES, pH 7.5, was used to equilibrate the column and elute any protein that did not bind to the column. Buffer B had the same composition as buffer A but contained 0.3 m imidazole for competitive elution. Non-specifically bound protein was eluted with 15 mm imidazole. Protein bound specifically through the His tag was eluted with a 25-ml gradient from 15 mm imidazole to 120 mm imidazole, programmed on the fast performance liquid chromatography system. The protein was collected in 8 or 9 fractions of 1 ml each and dialyzed against the same 55% glycerol buffer as used for the untagged myosin. Measurements were made within a week of preparation; longer storage at -20 °C resulted in gradual loss of function.
Skeletal muscle actin was prepared from chicken pectoralis acetone powder essentially as described (12) and stored at 4 °C as F-actin (10-15 mg/ml) in 5 mm KCl, 5 mm imidazole, pH 7.5, 2 mm MgCl2, 3 mm NaN3. It was used within 2-3 weeks of preparation.
S1 Preparation—The His-tagged myosin preparation was reacted with 0.3 mm MgATP to dissociate any actomyosin and clarified by centrifugation. After overnight dialysis against 20 mm HEPES, pH 7.0, 0.12 m NaCl, 1 mm EDTA, 1 mm NaN3, and 0.2 mm DTT, 1 mg/ml chymotrypsin (dissolved in 1 mm HCl) was added dropwise to the myosin suspension at room temperature to a final concentration of 0.06 mg/ml and stirred for 15 min. The digestion was stopped with 2 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride. The myosin digest was centrifuged to pellet-undigested myosin and rod, and the supernatant, containing mainly S1, was loaded onto the HiTrap chelating HP column. Buffers A and B were identical to the buffers used to purify His-tagged myosin on the same chelating column. Non-specifically bound S1 was eluted at 30 mm imidazole, and the His-tagged S1 was eluted by stepping the imidazole concentration to 120 mm. The protein was collected in ∼3 fractions of 1 ml each and dialyzed versus 55% glycerol buffer containing 20 mm KCl, 20 mm imidazole, pH 7.5, 1 mm EGTA, 1 mm MgCl2, 1 mm NaN3, and 1 mm DTT for storage at -20 °C.
When S1 was prepared from untagged-myosin, the myosin was first purified using the Toyopearl column as described above. After several hours of dialysis to reduce the ammonium sulfate concentration, the myosin was dialyzed overnight versus 20 mm imidazole, pH 7.0, 0.12 m NaCl, 1 mm EDTA, 1 mm NaN3, and 0.5 mm DTT before digestion with chymotrypsin. Digestion conditions leading to S1 were the same as for His-tagged myosin.
Actin-activated ATPase—The actin-activated MgATPase activities for S1 isoforms were determined at 30 °C in 5 mm NaCl, 10 mm imidazole, pH 7.0, 1 mm MgCl2, 1 mm NaN3, and 1 mm DTT. Actin concentrations ranged from 1 to 100 μm. The reactants were vigorously stirred in a total volume of 400 μl to ensure a homogeneous solution. For the myosin isoforms, the salt concentration was increased to 50 mm NaCl for better mixing, and the maximum actin concentration was reduced to 40 μm because of the greater actin affinity of two-headed myosin. All other conditions remained the same as for S1. The data were fit to Michaelis-Menten kinetics to determine Vmax and Km; see Trybus (13) for details.
In Vitro Motility Assay—In vitro motility was performed at 30 °C, essentially as described previously (11). The basic motility buffer contained 25 mm KCl, 25 mm imidazole, pH 7.5, 4 mm MgCl2, 1 mm EGTA, and 10 mm DTT. Myosin (0.3 mg/ml) in motility buffer (but with a higher salt concentration of 0.6 m KCl) was mixed with a 2-fold molar excess of F-actin and 1 mm MgATP and centrifuged for 25 min at 400,000 × g in the Optima Max-E ultracentrifuge to remove any myosin heads that are unable to dissociate from actin in the presence of ATP, the so-called “rigor heads.” This step was omitted for hydrophobic interaction chromatographed myosin, which has few if any rigor heads (11). The myosin (∼70 μg/ml) was then added to the flow cell and adsorbed directly onto nitrocellulose-coated coverslips for 60 s. Two rinses of 0.5 mg/ml bovine serum albumin in motility buffer were added to block the surface. An actin wash with fragmented, unlabeled actin was then used to further block rigor heads as described in Palmiter et al. (14). Briefly, unlabeled F-actin (1 μm) was fragmented by vortexing for 1 min, introduced into the flow cell in the absence of ATP, and incubated for 30 s. 1 mm MgATP in motility buffer was used to release the actin from functional myosin heads, leaving only rigor heads blocked followed by rinsing with motility buffer to remove the MgATP. Phalloidin rhodamine-labeled F-actin was then added to the flow cell for 30 s and rinsed with motility buffer. The assay was performed in motility buffer with 1 mm MgATP, 0.5% methylcellulose, and an oxygen scavenging system (3 mg/ml glucose, 0.1 mg/ml glucose oxidase, 0.18 mg/ml catalase). Actin movement was observed using an inverted microscope (Zeiss Axiovert 10) equipped for epifluorescence, with an image intensified CCD camera (Hamamatsu C2400-80), detailed in Trybus (13).
Motility data were recorded on a VCR and then digitized for analysis, with a sampling rate of 10 frames/s. Data were analyzed using a semi-automated filament tracking program, described in Kinose et al. (15). The program automatically determines the trajectory of each actin filament in the data set with a non-zero velocity and movement lasting at least 10 frames. Each data set yields a weighted distribution of filament velocities, which is fit with a Gaussian to determine the mean velocity and S.D. This type of analysis allows hundreds of filaments to be tracked and allows the entire field to be characterized without selection bias. Filaments were also tracked manually as described in Work and Warshaw (16). The mean velocities obtained from the two techniques agreed to within 10%.
Protein Concentration—The protein concentration was determined by measuring absorbance at 280 nm using an extinction coefficient (1 mg/ml, 1-cm path length) of 0.55 for myosin, 0.75 for chymotryptic S1, and 0.63 for actin at 290 nm. Any light scattering at 320 nm was subtracted from the absorbance at 280 nm. Protein concentration was also determined by the Bradford assay (Pierce) using chicken skeletal myosin or bovine serum albumin as the standard. The two methods gave excellent agreement.
Gel Electrophoresis—Precast 12% Bis-Tris gels (Invitrogen) were used to monitor the steps in the myosin and S1 preparations. Precast 3-8% Tris acetate gels (Invitrogen) were used for Western analysis with an anti-His monoclonal primary antibody (Sigma). A His-tagged recombinant smooth muscle myosin was used as a standard to determine the amount of His-α-MHC. Bis-Tris/SDS gels containing 5% glycerol and 8% acrylamide were used to separate the α- and β-MHC isoforms in mouse cardiac myosin (8). Several loads of protein (in the 0.5 μg range) were loaded on a mini gel system and run at a constant current of 20 mA for ∼2 h. The duration of the run was determined by the migration of the 250-kDa band in the Pre-Stained Standard (Invitrogen) used as a marker.
The relative amount of isoform was determined by densitometry of the intensity of each myosin band using the Kodak Gel Logic 100 Imaging System. Four different loads of protein could be fit with a straight line that went through the origin. The ratio of the slopes was used to determine the % β-MHC.
RESULTS
The cDNA encoding the mouse β-MHC gene and the mouse α-MHC gene was cloned into the α-MHC promoter cassette, which contains the mouse α-MHC promoter upstream and a human growth hormone (hGH) polyadenylation signal downstream, Fig. 1A. A His6-epitope tag was introduced at the N terminus to facilitate isolation by metal chelating chromatography, and the R403Q mutation was cloned into the MHC for both α- and β-cardiac myosin isoforms. Wild-type MHC constructs also had the His tag for the purpose of serving as a control for the mutants. As shown in Fig. 1B, the amount of transgenic α-myosin cannot be determined in 5% glycerol, SDS gels because His-tagged protein co-migrates with untagged, endogenous α-myosin. However, the TG β-myosin is well separated from endogenous mouse α-myosin and has the same mobility as rabbit β-myosin. The R403Q mutation is located at the base of a surface loop that has been shown to interact with actin for several myosin isoforms (Refs. 17 and 18 and Fig. 1C).
Isolation and Characterization of TG Mouse α-MHC—We assumed that the His-tagged α-MHC (at ∼50% expression levels) assembles randomly with the untagged, endogenous α-MHC to give a population of 1:2:1 molecules of untagged, singly tagged, and doubly tagged α-myosin, respectively. When a crude preparation of myosin (see “Experimental Procedures”) was applied to a Ni2+-chelating column, Fig. 2, A and B, some contaminants including actin (probably in the form of actomyosin) and tropomyosin appeared in the flow-through, peak I, and some were retained by the column along with untagged α-myosin, peak II. These non-specifically bound proteins were eluted with 15 mm imidazole. The application of a gradient from 15 to 120 mm imidazole eluted purified His-tagged myosin with only a trace of actin, peak III. Western blotting confirmed that peak II was non-specifically bound untagged protein, Fig. 2C. The bound protein, peak III, consisted of singly His-tagged myosin along with some doubly His-tagged myosin. The shoulder on peak III suggests a slight separation of singly His-tagged from doubly His-tagged molecules but not enough to isolate a pure doubly His-tagged species. This separation profile is typical for all the myosin preparations we used, except for variations in absorbance among the three peaks.
FIGURE 2.
Isolation of His-tagged α-cardiac myosin using a Ni2+-chelating column. A, about 16 mg of crude myosin isolated from a TG mouse line expressing R403Q α-MHC was applied (in ∼4 ml) to a 5-ml HiTrap chelating HP column (Amersham Biosciences). After the flow-through (peak I), non-specifically bound protein was eluted with 15 mm imidazole (peak II), and the specifically bound myosin was eluted with an imidazole gradient from 15 to 120 mm (peak III) in 5 column volumes (25 ml). The total yield from the front and back of the peak after dialysis against 55% glycerol was ∼4 mg. Concentrated pooled fractions from the load, and peaks are shown in the 12% SDS gels (B) and the corresponding Western blot (C) using an antibody specific for the His6 tag. The protein eluted at 15 mm imidazole is unreactive with the antibody, indicating the absence of a His tag. The chromatogram and corresponding gels shown here did not change significantly with isoform type or the presence of a mutation. mAU, milliabsorbance units; TM, tropomyosin; RLC, regulatory light chain; ELC, essential light chain.
To determine the effect of the R403Q mutation on the movement of actin filaments by mouse α-myosin, we compared His α-MHC(R403Q) to His α-MHC(WT) in the in vitro motility assay, Fig. 3 and Table 1. Recognizing that the mutant preparation may be predominantly heterodimeric myosin (i.e. only one head contains the R403Q mutation), the ∼22% increase in motility compared with WT is quite consistent with published values for homozygous R403Q α-myosin (7, 19). The small variations in actin filament velocity among wild-type or mutant preparations, Table 1, are well within the experimental error of the method. The semi-automated filament tracking program (15, 20) used here records the movement of several hundred filaments without selection bias. Stationary filaments are not included, but greater than 95% of the field moved well. The mean velocity obtained by this analysis agreed to within 10% with the mean value of 40-50 filaments selected manually and analyzed by programs used previously (16). Irrespective of the method of analysis, the velocity for the mutant α-myosin (5.0-5.5 μm/s) was consistently higher than the wild-type α-myosin (4.0-4.6 μm/s) measured in parallel.
FIGURE 3.
Actin filament velocities for His-tagged α-cardiac myosin. A, wild-type. B, R403Q mutant. Histograms of actin filament velocities from the in vitro motility assay. Each histogram is fit with a Gaussian to obtain the mean velocity and S.D. The number of moving filaments is n. One representative data set is shown here for each myosin (Table 1, experiment 2). The back half of peak III, which is expected to be enriched in the homodimeric population of His-tagged molecules, was used for the mutant myosin.
TABLE 1.
In vitro motility of WT and mutant α-cardiac myosin
The His-tagged protein from transgenic mice was purified by metal chelation chromatography, whereas the untagged-myosin from non-transgenic mice was purified by hydrophobic interaction chromatography.
| Sample | Experimenta | Motilityb | Motilityc |
|---|---|---|---|
| μm/s | μm/s | ||
| His α-MHC (WT) | 1 | 3.9 ± 0.4 | 3.6 ± 0.5 |
| His α-MHC (WT) | 2 | 4.1 ± 0.7 | 3.7 ± 0.6 |
| His α-MHC (WT) | 3 | 4.6 ± 0.5 | 4.4 ± 0.7 |
| His α-MHC (403) | 2 | 5.0 ± 0.7 | 4.7 ± 0.7 |
| His α-MHC (403) | 3 | 5.5 ± 0.6 | 6.0 ± 0.9 |
| Untagged α-MHC | 4 | 3.8 ± 0.4 | |
| Untagged α-MHC | 5 | 4.4 ± 0.5 |
The same experimental number means that WT and mutant samples were prepared and measured within a week of each other.
Analysis with a semi-automated filament tracking program (15).
Manual analysis of at least 40-50 actin filaments per experiment (16). Both sets of motility values are reported as the mean ± S.D.
Expression and Characterization of TG Mouse β-MHC—Expression of wild-type His-tagged β-MHC in transgenic mice resulted in replacement levels of 60% or more of the total cardiac MHC (Fig. 4A, lane 1). This compares favorably with replacement levels for untagged β-myosin reported previously (8), demonstrating that the His tag has no detrimental effect on protein expression in mouse hearts. Fractionation of the total extracted myosin on a Ni2+-chelating column, as described above, yielded myosin considerably enriched in His β-MHC (WT) from the back portion of peak III (Fig. 4A, lane 2). The amount of residual α-myosin seen in the separation gels (<10%) is comparable with that present in mice treated with PTU for 11 weeks (see Ref. 8 and Fig. 6, inset). The motility of wild-type His β-MHC (∼2.4 μm/s) is also the same as that of the mouse β-MHC produced by PTU treatment for 11 weeks (Refs. 10 and 11, Fig. 4B, and Table 2).
FIGURE 4.
Isolation and velocity of His-tagged β-cardiac myosin. Myosin from transgenic mice in which the endogenous ventricular α-MHC was replaced to varying degrees with His-tagged β-MHC was eluted in peak III from the same type of metal chelating column as described in Fig. 2. A, 5% glycerol gels showed about 60% replacement of the endogenous α-myosin with expressed wild-type β-MHC (lane 1) but only about 10% replacement with expressed R403Q mutant (lane 3). Isolation of myosin from the back of peak III increased the amount of WT β-MHC to ∼90% (lane 2), and the amount of R403Q mutant was ∼50% (lane 4). B, the actin filament velocity for the largely homodimeric WT β-myosin was consistently about 2.4 μm/s, as shown in this representative data set, but the velocity for the α/β-MHC(R403Q) heterodimer was closer to 4 μm/s (see Table 2, experiment 2).
FIGURE 6.
Isolation and velocity of β-cardiac myosin from PTU-treated mice by hydrophobic interaction chromatography. A, about 7 mg of crude myosin was applied to a 5-ml prepacked Toyopearl MD-G ether column (1 × 6.8 cm, TosoHaas) equilibrated in 1.4 m ammonium sulfate. A step to 1.2 m ammonium sulfate eluted the protein in 3-4 1-ml fractions, which were concentrated by dialysis against 55% glycerol to yield ∼1.2 mg myosin in 1 ml. Inset, mice given a PTU diet for 11 weeks had >90% replacement of α- with β-MHC, whereas mice treated for 5 weeks with PTU showed only 75% replacement on 5% glycerol gels. B, the difference in motility, 3.2 μm/s (5 weeks) versus 2.5 μm/s (11 weeks), reflects the greater proportion of α-myosin in the ventricles at 5 weeks (Table 2, experiment 6). mAU, milliabsorbance units.
TABLE 2.
In vitro motility of WT and mutant β-cardiac myosin
The His-tagged β-myosin isoform was isolated from transgenic mice, whereas the untagged β-myosin was isolated from PTU-treated mice.
| Sample | Experimenta | Motilityb | % β-MHCc |
|---|---|---|---|
| μm/s | |||
| HIS β-MHC (WT) | 1 | 2.4 ± 0.4 | 83 |
| HIS β-MHC (WT) | 2 | 2.4 ± 0.4 | 86 |
| HIS β-MHC (WT) | 3 | 2.4 ± 0.5 | 82 |
| HIS β-MHC (403) | 2 | 4.0 ± 0.6 | 43 |
| HIS β-MHC (403) | 3 | 3.6 ± 0.5 | 54 |
| 2.8 ± 0.4 | 64 | ||
| HIS β-MHC (403) | 4 | 3.5 ± 0.6 | 58 |
| 3.6 ± 0.8 | 65 | ||
| 3.4 ± 0.6 | 68 | ||
| 3.0 ± 0.6 | 72 | ||
| PTU β-MHC 5 weeks | 5 | 3.6 ± 0.6 | 76 |
| PTU β-MHC 5 weeks | 6 | 3.2 ± 0.5 | 73 |
| PTU β-MHC11 weeks | 6 | 2.5 ± 0.5 | 90 |
The same experimental number means that WT and mutant samples were prepared and measured within a week of each other.
All values were determined by the semi-automated tracking program (15) and are reported as the mean ± S.D. Samples of His-tagged WT represent pooled fractions from the back of peak III. Because of the low yield of the His-tagged 403 mutant in experiment 2, the entire peak III was pooled. Increasing the replacement level for the 403 mutant by cross-breeding (see “Experimental Procedures”) made it possible to analyze independent fractions across peak III in experiments 3 and 4.
The relative amount of β- to α-MHC isoform was determined by densitometry of several loads for each sample on 5% glycerol, SDS separation gels.
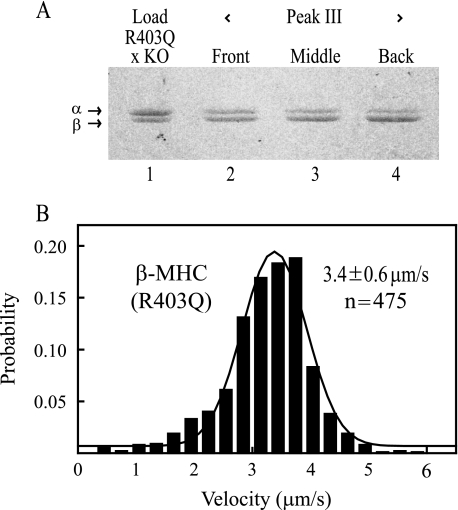
Unexpectedly, expression levels of mutant His-tagged β-MHC(R403Q) were very low, 10-15% of the total cardiac myosin (Fig. 4A, lane 3). The mortality rate of the mice was also higher than usual, and the surviving TG lines all expressed low levels of mutant protein. Fractionation on the chelating column yielded essentially a heterodimeric population of His-tagged β-MHC(R403Q)/untagged α-MHC molecules (Fig. 4A, lane 4). The actin filament velocity of this heterodimeric mutant was high (4.0 μm/s) but not much higher than what would be calculated for a heterodimer of equal numbers of independently cycling wild-type heads of β-MHC and α-MHC (∼3.5 μm/s). To increase the expression level of mutant β-MHC, the R403Q β-MHC mouse was bred with a mouse carrying only a single functional allele for the α-MHC (9), referred to here as R403Q × KO. This reduction in α-MHC gene product led to higher expression levels of the β-MHC(R403Q) gene, resulting in a significant increase of R403Q β-myosin (∼40%) in the total cardiac myosin extract (Fig. 5A, lane 1). When this preparation was applied to the Ni2+ column, further enrichment in R403Q β-MHC was achieved in the fractions across peak III (Fig. 5A, lane 4). The back of the peak (∼70% β-myosin) was used in the motility assay (Fig. 5B and Table 2), giving an actin filament velocity of 3.4 μm/s. The calculated velocity, again assuming independent WT α- and β-heads at a ratio of 30:70, respectively, would be ∼3.0 μm/s (0.3(4.4 μm/s) + 0.7(2.3 μm/s)). The calculated and observed motility values are sufficiently similar to conclude that the R403Q mutation has not affected the speed of the β-MHC heads. The assumption of independently cycling heads is supported by the finding that mouse α-MHC has the same force as mouse β-MHC (10, 11), unlike cardiac isoforms of higher mammals, which show a 2-fold difference in force and corresponding effects on velocities of mixtures (10).
FIGURE 5.
Characterization of His-tagged R403Q β-MHC isolated from mice cross-bred with an α-MHC knock-out mouse line (KO) to increase expression levels of R403Q. A, 5% glycerol gels showed about 40% replacement of α-myosin with R403Q β-MHC (lane 1). Fractions across peak III from the Ni2+-chelating column showed slightly increasing amounts of R403Q β-MHC from 60% replacement (lane 2) to 65% (lane 3) and 70% (lane 4). A concentrated fraction from the back of peak III was used in the motility assay (B). There was little difference in motility between the front of the eluted peak III, 3.5 μm/s, and the back, 3.4 μm/s, given the marginal ability of this column to separate singly His-tagged heterodimeric myosin from doubly His-tagged homodimeric myosin (Table 2, experiment 4).
Properties of β-Myosin Isolated from PTU-treated Mice—Mice made hypothyroid by a diet of PTU show a rapid conversion from the endogenous α-MHC to β-MHC in their ventricles. This isoform switch is usually thought to be complete by 6-8 weeks. The original purpose in preparing β-myosin by PTU treatment was to demonstrate that the His tag had no deleterious effect on the functional properties of β-myosin. To purify the untagged myosin, we used hydrophobic interaction chromatography, which we have shown previously to be extremely effective in preparing highly purified myosin from small amounts of tissue (Fig. 6A and Ref. 11). Unexpectedly, myosin isolated from 5-week-old mice showed ∼25% α-MHC on separation gels (Fig. 6A, inset) and moved actin filaments with a velocity of 3.2 μm/s in the motility assay (Fig. 6B). By treating the mice with PTU for 11 weeks, the more typical preparation of β-myosin was obtained, with ∼10% α-myosin and a velocity of 2.5 μm/s (Fig. 6B and Table 2, experiment 6). This experiment proved that the motility of the transgenic WT β-myosin was identical to that of the PTU-derived myosin when the contamination level with α-myosin was <10% for both. Thus, there is no discernible effect of the His tag on the motility; the same was found to be true for the ATPase activity (see below). But even more interesting, the motility of the 5-week-old PTU-derived β-myosin, 3.2 μm/s, was essentially the same as that of the transgenic mutant β-MHC(R403Q), 3.4 μm/s, each containing 25-30% α-MHC (Table 2). The implication of these results is that the R403Q mutation does not enhance movement in β-cardiac myosin.
Actin-activated MgATPase Activity of Myosin S1 Isoforms—A complication in interpreting the motility data for TG β-myosin is the presence of two heads with different cycling rates in the dimeric myosin molecule. Unfortunately, it is not possible to isolate a homodimeric R403Q β-myosin due to low expression levels. The only available solution to this problem is to generate S1 heads by proteolytic digestion of myosin and then isolate the His-tagged R403Q heads by affinity chromatography (Fig. 7), as described for myosin. The course of the digestion with α-chymotrypsin is shown in Fig. 7B. The insoluble residual myosin and rod are pelleted by centrifugation, and the supernatant contains S1 and minor contaminants. The untagged α-S1 (peak II) and the tagged-β-S1 (peak III) are readily separated at different imidazole concentrations on the Ni2+-column (Fig. 7, A and C). The only drawback to S1 is the inability to do motility assays. For kinetic assays, however, S1 is the preparation of choice.
FIGURE 7.
Preparation of His-tagged S1 using a Ni2+-chelating column. Crude myosin was digested with α-chymotrypsin (see “Experimental Procedures”) to yield His-tagged S1. About 8 mg of S1 was applied to a HiTrap chelating column as described above for myosin. A, the non-specifically bound S1 eluted at 30 mm imidazole (a Western blot showed no reactivity for peak II with the anti-His antibody), and the His-tagged S1 was eluted with a step to 120 mm imidazole (peak III). About 0.7-0.8 mg of purified S1 in 1 ml was recovered after dialysis against 55% glycerol. mAU, milliabsorbance units. B, the SDS gel shows that the myosin (lane 1) was digested to completion resulting in rod and S1 heavy chain (lane 2). The regulatory light chain (RLC) is degraded by the enzyme. The insoluble rod is pelleted by centrifugation (lane 3), and the supernatant (Sup., lane 4) contains primarily S1 and some contaminating tropomyosin. C, SDS gels of samples from the column (peaks identified at top of the gels) show the breakthrough (lane 5), untagged S1 (lane 6), and purified His-labeled S1 (lane 7). ELC, essential light chain.
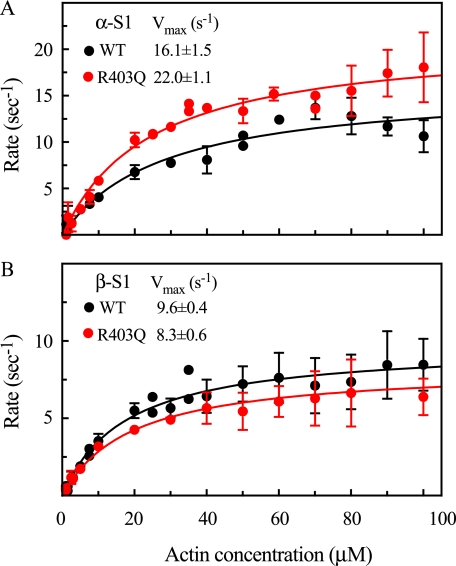
The kinetic results from two independent preparations (a minimum of 10 hearts for each preparation) of wild-type and mutant α- and β-S1 isoforms are summarized in Fig. 8 and Table 3. The actin-activated MgATPase activity (Vmax) for the α-S1(R403Q) mutant is ∼30% higher than α-S1(WT) (Fig. 8A). This difference is statistically significant (p < 0.05). The level of enhancement is in good agreement with the motility results for the α-myosin isoform presented here. In contrast, the β-S1(R403Q) isoform showed no enhanced ATPase activity. In fact, the individual preparations usually showed a slightly lower activity for the mutant compared with wild type, and the fit to all the data indicated a reduction of ∼10% in activity, but this difference is not statistically significant (Fig. 8B). These data are quite consistent with the motility results (Figs. 5 and 6), which suggested that the mutation had little effect on movement under unloaded conditions. It should be noted that the Vmax for wild-type α-S1 is ∼1.7 times β-S1, maintaining the 2-fold difference in activity found for the myosin isoforms (2.5 s-1 versus 1.4 s-1 for α-MHC and β-MHC, respectively; supplemental Fig. S1). The ∼6-fold higher Vmax values for the S1 subfragments compared with myosin have been found for many striated muscle myosin IIs, including fast skeletal myosin (21) and slow/cardiac myosin (22). The increase in activity is probably due to the loss of the regulatory light chain during chymotryptic digestion, which results in a loss of regulation at the active site. Although there are substantial uncertainties in these measurements, particularly at the higher actin concentrations where the high viscosity of F-actin precludes good mixing, the consistency of the measurements over several independent heart preparations provides a high level of confidence in the results.
FIGURE 8.
Actin-activated MgATPase activities for mouse wild-type and R403Q mutant α- and β-S1. Most data points represent an average of three or more determinations (in some cases the error bars are smaller than the symbols) for a minimum of two independent protein preparations. The error bars represent S.D. The data were fit to the Michaelis-Menten equation to obtain the Vmax and Km values reported below with the S.E. A, wild-type α-S1 has a Vmax of 16.09 ± 1.47 s-1 and a Km of 27.90 ± 7.20 μm. The R403Q mutant has a Vmax of 22.03 ± 1.12 s-1 and a Km of 28.06 ± 4.00 μm. B, wild-type β-S1 has a Vmax of 9.56 ± 0.39 s-1 and a Km of 16.90 ± 2.39 μm. The R403Q mutant has a Vmax of 8.30 ± 0.61 s-1 and a Km of 19.04 ± 4.86 μm.
TABLE 3.
ATPase activity of wild-type and mutant cardiac myosin subfragment-1 isoforms
| Sample | Experimenta | Vmax | Km |
|---|---|---|---|
| s−1 | μm | ||
| His α-S1 (WT) | 1 | 11.0 ± 1.2 | 15.9 ± 3.7 |
| 2 | 15.0 ± 1.3 | 20.0 ± 6.9 | |
| His α-S1 (403) | 1 | 23.3 ± 1.4 | 29.6 ± 3.8 |
| 2 | 22.1 ± 2.6 | 29.7 ± 11.3 | |
| His β-S1 (WT) | 3 | 9.3 ± 0.6 | 15.5 ± 2.9 |
| PTU β-S1 | 4 | 10.8 ± 0.9 | 24.0 ± 6.1 |
| His β-S1 (R403Q) | 3 | 7.8 ± 0.7 | 17.1 ± 3.7 |
| 4 | 7.2 ± 0.6 | 11.7 ± 4.6 |
The same experimental number means that wild-type and mutant samples were prepared and measured within a week of each other. Vmax and Km are reported with the S.E.
DISCUSSION
The discovery that a point mutation, R403Q, in the β-MHC could lead to hypertrophic cardiomyopathy occurred nearly two decades ago (1), but despite the large number of subsequent in vitro and in vivo studies, even the most elementary question of how this point mutation affects the functional properties of cardiac myosin has remained unresolved. The majority of studies in the decade after the original discovery prepared myosin from biopsies of patients with FHC (23, 24) or from a variety of expression systems (25-28). With the introduction of the mouse model in 1996 (6), attention shifted to myosin isolated from homozygous (R403Q/R403Q) mouse hearts. Unlike previous findings, which showed mainly a loss of function due to FHC mutations, the mouse R403Q myosin showed a large increase in actin filament velocity as well as enzymatic activity (7). The enhanced function was attributed to improvements in the quality of the myosin as well as technical advances. Because the R403Q mutation in recombinant smooth muscle myosin also showed increased motility and ATPase activity (29), it was hypothesized that the functional effects of the mutation are probably independent of myosin isoform type and apply to all members of the myosin class II superfamily (for review, see Ref. 5). Here we show that this tacit assumption is not necessarily correct; the introduction of the R403Q mutation does indeed enhance the enzymatic and mechanical properties of mouse α-cardiac myosin as proposed earlier, but this effect is not observed in mouse β-cardiac myosin, which shows no gain of function under identical experimental conditions.
Properties of Mouse α-Cardiac Myosin (R403Q)—The effects of a disease-causing point mutation on protein structure and function are usually not large, and therefore, a mouse homozygous for the lethal R403Q point mutation was initially chosen to assure the preparation of a homodimeric cardiac myosin for biophysical studies (7). The large (∼60%) increase in actin filament velocity (Vactin) for R403Q α-cardiac myosin compared with wild type was unexpected given the long history of diminished function in a number of different myosins. A more recent study that focused on the force-generating properties of this homodimeric R403Q α-myosin reported a more modest increase in the actin filament velocity (24%), but the basic theme of enhanced mechanical performance was preserved (19).
Here our aim was to characterize a highly purified preparation of mutant α-cardiac myosin not only to confirm the published results but to provide a set of functional measurements against which the properties of β-cardiac myosin carrying the same mutation could be compared. To purify myosin by any suitable chromatographic procedure, one needs to start with a minimum amount of protein. The hearts from 1-week-old mice homozygous for R403Q weigh only ∼20 mg, and a single heart typically yields only about 0.2 mg of protein. The cloning of the His6 tag at the N terminus of the MHC made it possible to start with ∼10 mature heterozygous mouse hearts (∼1 g tissue), which was ample material to isolate ∼4 mg of myosin by metal chelating chromatography. A minimum of a 20% increase in Vactin was found for the isolated R403Q α-cardiac MHC compared with WT; if this myosin fraction contained mainly heterodimeric molecules, the calculated Vactin for a homodimeric molecule would increase by 30-40%. This level of enhancement due to the R403Q mutation falls well within the range of values reported for R403Q α-MHC from homozygous mice (7, 19).
Although myosin isolated from a homozygous mouse is adequate for motility assays (provided all rigor bonds are removed by an actin wash in the flow cell; see “Experimental Procedures”), there is insufficient protein to prepare a soluble subfragment for ATPase activity measurements. A great advantage of the His tag is the ability to isolate single heads (S1) containing the mutation, as the tag is cloned along with the mutation on the same MHC. Kinetic studies of the cross-bridge cycle have been done exclusively with soluble subfragments to avoid the difficulties associated with mixing filamentous myosin and F-actin at the low salt conditions required for the ATPase assay. The increase (∼30%) in the steady-state actin-activated ATPase of R403Q α-S1 compared with wild-type S1 is remarkably consistent with the increase in actin filament velocity. Although the velocity of unloaded muscle shortening (and the analogous in vitro translocation of actin filaments by myosin) has been shown to be proportional to actomyosin ATPase activity (30, 31), this coupling does not necessarily extend to mutant myosins (32). However, in the case of the R403Q mutation there does appear to be a linear relationship between the enzymatic and mechanical properties.
Properties of Mouse β-Cardiac Myosin (R403Q)—The first clue that mutant β-cardiac myosin (R403Q) may have different functional properties from α-cardiac myosin (R403Q) came from the difficulties encountered in expressing the transgene in mice. Unlike α-MHC (R403Q), which readily replaced up to 50% of the endogenous cardiac myosin without any overt detrimental effects on the animals, the mice expressing the mutant β-MHC gene had low survival rates, and the amount of myosin replaced barely exceeded 15%. Multiple injections were attempted to generate transgenic lines with increased levels of replacement, but to no avail. This poor outcome was not caused simply by an isoform shift, as wild-type β-myosin could replace the endogenous α-cardiac myosin by >70% with no ill effects (8) (there is no “overexpression” of a protein in sarcomeric protein transgenesis; rather, the level of endogenous protein is down-regulated and replaced proportionally by the transgenic protein). An insight into how such a small amount of β-MHC(R403Q) protein could have such a profound influence on mortality and isoform expression has come from recent studies showing that the cardiac isoform distribution is not uniform throughout the adult mouse heart (33). Rather, the small amount of native β-MHC (<7%) normally present in adult mice was localized by immunofluorescence to discrete regions of the heart (33). It is conceivable, therefore, that a small amount of mutant β-MHC concentrated in a critical region of the heart could have an unusually large functional impact.
Despite the low level of R403Q β-MHC expression, the presence of the His tag ensured that at least half the heads in affinity-purified myosin were mutated. The motility of this heterodimer (R403Q β-MHC/α-MHC) was similar to transgenic wild-type α-MHC, about 4 μm/s. However, the interpretation of motility for a heterodimeric myosin with heads cycling at two different rates is ambiguous; if the mutant head was compromised and silent, the net velocity would be the same as if the mutant β-head had increased its cycling rate. By cross-breeding the His-tagged R403Q mouse with an α-MHC knock-out mouse line (9), it was possible to isolate a mutant population with about ∼70% R403Q β-MHC and 30% endogenous α-MHC. By a serendipitous circumstance, we also prepared a control myosin with 75% β-MHC from mice that had undergone an incomplete, hormonally induced isoform shift. The sliding velocity of this PTU-induced native β-myosin was essentially the same as the transgenic R403Q β-myosin, leading to the conclusion that the R403Q mutation did not have a significant effect on the unloaded sliding velocity of β-myosin heads. Moreover, the slight increase in motility observed for the non-transgenic β-myosin, which contained a population of α/β-MHC heterodimers, compared with the motility calculated for independently cycling α- and β-myosin heads, suggests that a small degree of intramolecular head/head interaction might affect the cycling rates (34).
Ambiguity in the interpretation of mechanical data for mixed cardiac myosin isoforms can be avoided in the analysis of enzymatic data for cardiac myosin subfragment-1. The presence of the His tag ensures a homogeneous population of mutant myosin heads. Measurements of three independent preparations of R403Q β-S1, each in parallel with wild-type β-S1, minimized experimental error due to variations in the activity of the S1 preparation. In contrast to R403Q α-S1, the ATP hydrolysis rate for R403Q β-S1 was either equal to or lower than the wild-type control but never higher. Given the large number of independent determinations, we believe that the ∼10% loss in average activity due to the mutation is meaningful (35). Thus, both the in vitro motility and the enzymatic experiments lead to the conclusion that the R403Q mutation does not enhance the functional properties of mouse β-cardiac myosin.
Comparison with Human R403Q Cardiac Myosin—The only viable source of R403Q β-myosin has been from the tissue of patients with FHC (23, 24). The small amount of protein obtained in a biopsy sample limits the functional assays to the in vitro motility assay. The soleus contains >80% slow (type 1) fibers, which primarily contain the same β-MHC as expressed in heart muscle. The β-myosin isoform could be separated from the fast myosin component by adsorption to a specific anti-β-MHC antibody bound to the coverslip of the flow cell. Both R403Q β-myosin from cardiac tissue and from the soleus muscle showed a large decrease (as much as 80%) in actin-sliding velocity compared with control values (23, 24). Considering that these patients are all heterozygotes, this marked inhibition suggests that the mutant myosin may have had more “rigor-like,” ATP-insensitive actomyosin linkages than the control samples. A subsequent motility study on cardiac biopsies from the same patients, in which great care was taken to remove any non-cycling rigor heads by exposure to unlabeled actin, showed a small enhancement in actin filament velocity for the R403Q β-myosin (14). These variable results emphasize the difficulties encountered in drawing conclusions from a single type of assay with an unstable, mutated protein.
This raises the question of whether results obtained with mouse β-cardiac myosin can be applied to human ventricular myosin. The 2-fold difference in functional properties between the α- and β-cardiac isoforms of the same species (∼93% sequence identity) has been largely attributed to clusters of non-identical residues located in loops at the actin-myosin interface (loop 2), near the nucleotide binding pocket (loop 1), the essential light chain binding lever arm, and certain regions of the myosin rod (10, 36). But the sequence of mouse β-MHC is ∼98% identical to human β-MHC, and even more importantly, the regional differences found between the α- and β-cardiac isoforms do not occur among β-isoforms from different species, where residue changes are primarily confined to conservative substitutions. The in vitro motility for mouse β-MHC appears to be slightly higher than for rabbit and pig β-MHC (2.4-2.6 μm/s versus 2.0 and 1.8 μm/s, respectively) (11), but these differences do not take into account the presence of 5-10% α-MHC in the PTU-induced β-MHC in the mouse compared with ∼100% β-MHC in mature, larger mammals (see the gel in Fig. 1). Therefore, the present evidence tends to favor the hypothesis that the results obtained for mouse β-MHC may well apply to the functional properties of higher mammals. Exceptions have been reported, however (37), and extrapolation of results from one species to another must be viewed with caution until more data become available.
Comparison with Muscle Fiber Studies—Are there any independent cellular experiments that can be correlated with the molecular studies on mutated α- and β-cardiac myosin? Isometric tension measurements on single slow fibers isolated from the soleus of patients with FHC (and analyzed for the ratio of mutant to wild-type β-MHC) showed a small (∼18%) but significant decrease in isometric tension for R403Q fibers compared with G741R, L908V, and control fibers (38). In a related study, isometric force and unloaded shortening velocity were both reduced in soleus fibers from FHC patients containing the R403Q and G741R β-MHC mutations (39).
Studies to examine the mechanical effects of the R403Q mutation in the mouse model have used skinned papillary muscle strips from a heterozygous α-MHC403/+ mouse generated by targeted recombination (6). Using small-amplitude length-perturbation analysis, depressed cross-bridge kinetics were observed for the mutant compared with wild-type, from which it was concluded that the R403Q α-MHC mutation reduces the strong binding affinity of myosin for actin (40, 41). A more recent study on skinned strips from the α-MHC403/+ mouse determined force-velocity relationships using the force-clamp technique (42). Maximum unloaded shortening velocity of the R403Q α-MHC muscle strips, as measured by the slack test, was significantly higher for the mutant than wild type, and the velocity of shortening under all loads was higher in the R403Q/+ α-MHC fibers. Thus, the mechanical power output was far greater for the mutant than for the control, indicating that the R403Q mutation in an α-MHC backbone enhances the mechanical performance of cardiomyocytes (42).
Conclusions and Future Perspective—We have shown that the effect of the R403Q mutation in a β-MHC backbone is different from the functional consequences in an α-MHC backbone in the mouse model for FHC. Thus, the contradictory results in the literature may arise to some extent from whether the source of the mutant myosin is from FHC patients (β-MHC) or from transgenic mice (α-MHC). The mechanical studies on muscle fibers containing α-and β-MHC are in general consistent with the in vitro molecular studies.
How can we explain these differential effects given that the surface loop containing the R403Q mutation (known as the cardiomyopathy loop) is identical in sequence for both α- and β-isoforms? Docking of the crystal structure of S1 into the three-dimensional reconstructions of the rigor actomyosin complex obtained by electron cryomicroscopy has shown that this loop interacts directly with actin (Fig. 1C) and forms part of an extensive actin-myosin interface that is conserved in several classes of myosin (17, 18, 43, 44). To study the structural consequences of the R403Q mutation, we used expressed smooth muscle S1 containing the mutation to “decorate” actin filaments for analysis by electron cryomicroscopy (mutant cardiac S1 was not available at the time this study was initiated). Unexpectedly, the three-dimensional reconstructions showed mutant smooth S1 attached to actin at highly variable angles compared with the usual fixed angle for wild-type S1, suggesting the mutation caused a severe disruption of the actin-myosin interface (45). Given the conserved nature of the cardiomyopathy loop and assuming that a similar disorder will be caused by mutant cardiac isoforms, one can speculate that the disorder at the actin interface may extend to the ionic interactions of the neighboring loop 2 with actin. It is well known that domains in the myosin molecule can communicate over long distances, and therefore, alterations at the actomyosin interface are likely to perturb the kinetics at the nucleotide binding pocket (Fig. 1C). Because cardiac α- and β-MHC have significant differences in sequence, it is plausible that the R403Q mutation should have contrasting effects on the functional properties of the two isoforms.
Recognizing that mouse and human β-cardiac myosin are probably not identical in function, the present findings apply strictly only to the transgenic mouse model for FHC. However, our study shows the kind of approach needed to attain a definitive answer for the human system. A transgenic rabbit model, with its endogenous β-MHC background, would be the best source for a mutant β-cardiac myosin. The currently available in vitro expression systems have failed to yield adequate amounts of a properly folded, functional cardiac myosin. A transgenic rabbit model has already been generated which expresses the human R403Q myosin heavy chain to levels of ∼40% that of the total β-MHC pool (46). By cloning a His tag at the N terminus of the MHC gene, it would be possible to purify mutated human myosin from mature transgenic rabbits to a high level of homogeneity for mechanical studies. Moreover, the His tag would make it possible for the first time to prepare a homogeneous mutant subfragment-1 from human cardiac myosin for kinetic measurements and structural analysis. We believe such studies would provide a definitive mechanism for how a point mutation in the human myosin heavy chain can trigger pathways that ultimately lead to a diseased heart.
Supplementary Material
Acknowledgments
We thank L. Martin, B. Silverstrim, and J. Dodge for excellent technical assistance with the transgenic mice. S. L. thanks U. Nair for providing the actomyosin model in Fig. 1C from coordinates in Volkmann et al. (17), K. M. Trybus for assistance with figures, D. A. Winkelmann for assistance with the semi-automated tracking program, and K. M. T. and D. M. Warshaw for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL69799, HL077101, HL087862, and HL074728 (to J. R.), AR053975 (to S. L.), HL59408 (to S. L. and J. R.), and P20 RR16435 (to S. W. and R. L.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: MHC, myosin heavy chain; FHC, familial hypertrophic cardiomyopathy; S1, subfragment-1; TG, transgenic; WT, wild-type; PTU, propylthiouracil; DTT, dithiothreitol; Bis-Tris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
References
- 1.Geisterfer-Lowrance, A. A., Kass, S., Tanigawa, G., Vosberg, H. P., McKenna, W., Seidman, C. E., and Seidman, J. G. (1990) Cell 62 999-1006 [DOI] [PubMed] [Google Scholar]
- 2.Seidman, J. G., and Seidman, C. (2001) Cell 104 557-567 [DOI] [PubMed] [Google Scholar]
- 3.Marian, A. J., and Roberts, R. (2001) J. Mol. Cell. Cardiol. 33 655-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tardiff, J. C. (2005) Heart Fail Rev. 10 237-248 [DOI] [PubMed] [Google Scholar]
- 5.Lowey, S. (2002) Trends Cardiovasc. Med. 12 348-354 [DOI] [PubMed] [Google Scholar]
- 6.Geisterfer-Lowrance, A. A., Christe, M., Conner, D. A., Ingwall, J. S., Schoen, F. J., Seidman, C. E., and Seidman, J. G. (1996) Science 272 731-734 [DOI] [PubMed] [Google Scholar]
- 7.Tyska, M. J., Hayes, E., Giewat, M., Seidman, C. E., Seidman, J. G., and Warshaw, D. M. (2000) Circ. Res. 86 737-744 [DOI] [PubMed] [Google Scholar]
- 8.Krenz, M., Sanbe, A., Bouyer-Dalloz, F., Gulick, J., Klevitsky, R., Hewett, T. E., Osinska, H. E., Lorenz, J. N., Brosseau, C., Federico, A., Alpert, N. R., Warshaw, D. M., Perryman, M. B., Helmke, S. M., and Robbins, J. (2003) J. Biol. Chem. 278 17466-17474 [DOI] [PubMed] [Google Scholar]
- 9.Jones, W. K., Grupp, I. L., Doetschman, T., Grupp, G., Osinska, H., Hewett, T. E., Boivin, G., Gulick, J., Ng, W. A., and Robbins, J. (1996) J. Clin. Investig. 98 1906-1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpert, N. R., Brosseau, C., Federico, A., Krenz, M., Robbins, J., and Warshaw, D. M. (2002) Am. J. Physiol. 283 H1446-1454 [DOI] [PubMed] [Google Scholar]
- 11.Malmqvist, U. P., Aronshtam, A., and Lowey, S. (2004) Biochemistry 43 15058-15065 [DOI] [PubMed] [Google Scholar]
- 12.Pardee, J. D., and Spudich, J. A. (1982) Methods Enzymol. 85 164-181 [DOI] [PubMed] [Google Scholar]
- 13.Trybus, K. M. (2000) Methods 22 327-335 [DOI] [PubMed] [Google Scholar]
- 14.Palmiter, K. A., Tyska, M. J., Haeberle, J. R., Alpert, N. R., Fananapazir, L., and Warshaw, D. M. (2000) J. Muscle Res. Cell Motil. 21 609-620 [DOI] [PubMed] [Google Scholar]
- 15.Kinose, F., Wang, S. X., Kidambi, U. S., Moncman, C. L., and Winkelmann, D. A. (1996) J. Cell Biol. 134 895-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Work, S. S., and Warshaw, D. M. (1992) Anal. Biochem. 202 275-285 [DOI] [PubMed] [Google Scholar]
- 17.Volkmann, N., Hanein, D., Ouyang, G., Trybus, K. M., DeRosier, D. J., and Lowey, S. (2000) Nat. Struct. Biol. 7 1147-1155 [DOI] [PubMed] [Google Scholar]
- 18.Volkmann, N., Ouyang, G., Trybus, K. M., DeRosier, D. J., Lowey, S., and Hanein, D. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 3227-3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debold, E. P., Schmitt, J. P., Patlak, J. B., Beck, S. E., Moore, J. R., Seidman, J. G., Seidman, C., and Warshaw, D. M. (2007) Am. J. Physiol. 293 H284-H291 [DOI] [PubMed] [Google Scholar]
- 20.Bourdieu, L., Magnasco, M. O., Winkelmann, D. A., and Libchaber, A. (1995) Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 52 6573-6579 [DOI] [PubMed] [Google Scholar]
- 21.Wagner, P. D., Slater, C. S., Pope, B., and Weeds, A. G. (1979) Eur. J. Biochem. 99 385-394 [DOI] [PubMed] [Google Scholar]
- 22.Tobacman, L. S., and Adelstein, R. S. (1984) J. Biol. Chem. 259 11226-11230 [PubMed] [Google Scholar]
- 23.Cuda, G., Fananapazir, L., Zhu, W. S., Sellers, J. R., and Epstein, N. D. (1993) J. Clin. Investig. 91 2861-2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuda, G., Fananapazir, L., Epstein, N. D., and Sellers, J. R. (1997) J. Muscle Res. Cell Motil. 18 275-283 [DOI] [PubMed] [Google Scholar]
- 25.Sweeney, H. L., Straceski, A. J., Leinwand, L. A., Tikunov, B. A., and Faust, L. (1994) J. Biol. Chem. 269 1603-1605 [PubMed] [Google Scholar]
- 26.Sata, M., and Ikebe, M. (1996) J. Clin. Investig. 98 2866-2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita, H., Sugiura, S., Momomura, S., Omata, M., Sugi, H., and Sutoh, K. (1997) J. Clin. Investig. 99 1010-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roopnarine, O., and Leinwand, L. A. (1998) Biophys. J. 75 3023-3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita, H., Tyska, M. J., Warshaw, D. M., Lowey, S., and Trybus, K. M. (2000) J. Biol. Chem. 275 28045-28052 [DOI] [PubMed] [Google Scholar]
- 30.Barany, M. (1967) J. Gen. Physiol. 50 (suppl.) 197-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyska, M. J., and Warshaw, D. M. (2002) Cell Motil. Cytoskeleton 51 1-15 [DOI] [PubMed] [Google Scholar]
- 32.Sherwood, J. J., Waller, G. S., Warshaw, D. M., and Lowey, S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 10973-10978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krenz, M., Sadayappan, S., Osinska, H. E., Henry, J. A., Beck, S., Warshaw, D. M., and Robbins, J. (2007) J. Biol. Chem. 282 24057-24064 [DOI] [PubMed] [Google Scholar]
- 34.Rovner, A. S., Fagnant, P. M., and Trybus, K. M. (2003) J. Biol. Chem. 278 26938-26945 [DOI] [PubMed] [Google Scholar]
- 35.Cumming, G., Fidler, F., and Vaux, D. L. (2007) J. Cell Biol. 177 7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNally, E. M., Kraft, R., Bravo-Zehnder, M., Taylor, D. A., and Leinwand, L. A. (1989) J. Mol. Biol. 210 665-671 [DOI] [PubMed] [Google Scholar]
- 37.Pereira, J. S., Pavlov, D., Nili, M., Greaser, M., Homsher, E., and Moss, R. L. (2001) J. Biol. Chem. 276 4409-4415 [DOI] [PubMed] [Google Scholar]
- 38.Malinchik, S., Cuda, G., Podolsky, R. J., and Horowits, R. (1997) J. Mol. Cell. Cardiol. 29 667-676 [DOI] [PubMed] [Google Scholar]
- 39.Lankford, E. B., Epstein, N. D., Fananapazir, L., and Sweeney, H. L. (1995) J. Clin. Investig. 95 1409-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchard, E., Seidman, C., Seidman, J. G., LeWinter, M., and Maughan, D. (1999) Circ. Res. 84 475-483 [DOI] [PubMed] [Google Scholar]
- 41.Palmer, B. M., Fishbaugher, D. E., Schmitt, J. P., Wang, Y., Alpert, N. R., Seidman, C. E., Seidman, J. G., VanBuren, P., and Maughan, D. W. (2004) Am. J. Physiol. 287 H91-H99 [DOI] [PubMed] [Google Scholar]
- 42.Palmer, B. M., Wang, Y., Teekakirikul, P., Hinson, J. T., Fatkin, D., Strouse, S., Vanburen, P., Seidman, C. E., Seidman, J. G., and Maughan, D. W. (2008) Am. J. Physiol. 294 H1939-H1947 [DOI] [PubMed] [Google Scholar]
- 43.Milligan, R. A. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 21-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkmann, N., Liu, H., Hazelwood, L., Krementsova, E. B., Lowey, S., Trybus, K. M., and Hanein, D. (2005) Mol. Cell 19 595-605 [DOI] [PubMed] [Google Scholar]
- 45.Volkmann, N., Lui, H., Hazelwood, L., Trybus, K. M., Lowey, S., and Hanein, D. (2007) PLoS ONE 2 e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marian, A. J., Wu, Y., Lim, D. S., McCluggage, M., Youker, K., Yu, Q. T., Brugada, R., DeMayo, F., Quinones, M., and Roberts, R. (1999) J. Clin. Investig. 104 1683-1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominguez, R., Freyzon, Y., Trybus, K. M., and Cohen, C. (1998) Cell 94 559-571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.