Abstract
Previously, it was shown that low-intensity focused ultrasound pulses applied along with an ultrasound contrast agent results in temporary blood-brain barrier (BBB) disruption. This effect could be used for targeted drug delivery in the central nervous system. This study examined the effects of burst length, pulse repetition frequency (PRF), and ultrasound contrast agent dose on the resulting BBB disruption. One hundred non-overlapping brain locations were sonicated through a craniotomy in experiments in rabbits (ultrasound frequency: 0.69 MHz, burst: 0.1, 1, 10 ms, PRF: 0.5, 1, 2, 5 Hz, duration: 20s, peak negative pressure amplitude: 0.1–1.5 MPa, Optison dosage 50, 100, 250 µl/kg). For each sonication, BBB disruption was evaluated using contrast-enhanced magnetic resonance imaging. The BBB disruption threshold (the pressure amplitude yielding a 50% probability for BBB disruption) was determined using probit regression for the three burst lengths tested. Tissue effects were examined in light microscopy for representative locations with similar amounts of contrast enhancement from each group. While changing the PRF or the Optison dosage did not result in a significant difference in the magnitude of the BBB disruption (P>0.05), reducing the burst length resulted in significantly less contrast enhancement (P<0.01). The BBB disruption thresholds were estimated to be 0.69, 0.47, and 0.36 MPa for 0.1, 1, and 10 ms bursts respectively. No difference was detected in histology between any experimental group. This data suggests that over the range of parameters tested, BBB disruption is not affected by PRF or ultrasound contrast agent dose. However, both the BBB disruption magnitude and its threshold depend on the burst length.
Keywords: Ultrasound, blood-brain barrier, drug delivery, ultrasound contrast agents, MRI
Introduction
Recently, several papers have described a method to temporarily disrupt the blood-brain barrier (BBB) using ultrasound pulses combined with a circulating ultrasound contrast agent (Hynynen et al. 2001; Yang et al. 2007; Sheikov et al. 2004; Hynynen et al. 2005; McDannold et al. 2005; McDannold et al. 2006; Hynynen et al. 2006; Kinoshita et al. 2006; Raymond et al. 2006; McDannold et al. 2007; Treat et al. 2007; Choi et al. 2007). Since these pulses can be applied at an ultrasound frequency that can readily be applied transcranially by a transducer located outside the body (Hynynen et al. 2005; Hynynen et al. 2006), the method represents a potentially noninvasive technique to produce targeted BBB disruption. This ability could facilitate the targeted delivery of drugs to the central nervous system.
The BBB is a functional and structural barrier in the vasculature of the central nervous system that limits or excludes the use of most therapeutic and imaging agents (Abbott and Romero 1996). It makes the development of new drugs difficult and prevents the use of many drugs that are effective in other parts of the body. Several strategies have been proposed or tested to circumvent the BBB, including creating drugs or drug carriers that can penetrate the barrier (Pardridge 2002), directly infusing agents via a catheter implanted in the brain tissue (Bobo et al. 1994), implanting devices that slowly release drugs to the brain tissue after surgery (Guerin et al. 2004), and introducing a catheter into the brain vasculature and infusing a solution such as mannitol to produce diffuse disruption of the BBB downstream (Doolittle et al. 2000; Neuwelt et al. 1979). Focused ultrasound offers several advantages over these methods: it is noninvasive, targeted, and does not necessarily require the development of new drugs. By steering the beam to multiple locations, one could potentially conform the area where the BBB is disrupted only in a desired target volume, thereby avoiding dose-limiting side effects. Alternatively, one may be able steer the beam to cover the entire brain, if desired.
The mechanism by which ultrasound causes BBB disruption is currently unknown. Presumably, it is related to the interaction between the ultrasound beam, the preformed microbubbles that make up the ultrasound contrast agent, and the vessel walls. Previous studies have indicated that it is at least partially a physiological response in contrast to simply a direct physical modification of the endothelial cells or the tight junctions between them (Sheikov et al. 2004; Raymond et al. 2006). Other work has suggested that the mechanism is not inertial cavitation (McDannold et al. 2006) and that the exposure levels are substantially below the threshold for bulk heating-induced effects (Hynynen et al. 2001).
In order to further understand the mechanisms for BBB disruption and to optimize the procedure, the present study was performed to investigate the effects of different ultrasound parameters. We investigated the effects of ultrasound frequency, burst length, pulse repetition frequency, and dosage of contrast agent on the threshold and magnitude of the BBB disruption and the associated tissue effects.
Methods
Animals
The experiments were approved by our institutional animal committee. Sonications were targeted at two non-overlapping locations in each hemisphere of the brains of male New Zealand white rabbits (weight: approximately 4 kg). The targets were one cm deep in the thalamus and approximately 3 mm lateral to the midline. The animals were anesthetized using IM injections of a mixture of 12 mg of sodium xylazine (Xyla-ject; Phoenix Pharmaceuticals, St Joseph, MO, USA) and 48 mg of ketamine hydrochloride (Abbott Laboratories, North Chicago, IL, USA) given per kg of body weight per hour. In order to reduce the uncertainty of the ultrasound exposure estimates in the brain, the sonications were not delivered transcranially; a craniotomy (approximately 2×2 cm) performed before the experiments provided an acoustic path into the brain. After the bone section was removed, the overlying skin was sutured and allowed at least two weeks so it could heal completely. The fur on the skin overlying the craniotomy was removed with hair clippers then depilatory lotion immediately before the experiments. A total of 104 locations were sonicated over the course of the experiments in 26 rabbits. Four locations were excluded from the analysis due to targeting errors.
Ultrasound
An air-backed spherically curved transducer (center frequency: 690 kHz; diameter/radius of curvature: 10/8 cm) generated the ultrasound beam (piezoelectric crystal purchased from Staveley Sensors, E. Hartford, CT; housing and cabling assembled in-house). This frequency was chosen as it can be used trans-cranially (Hynynen et al. 2005; Hynynen et al. 2006; McDannold et al. 2006) and is used in a prototype clinical device for noninvasive ablation of brain tumors (Hynynen et al. 2004). The transducer was driven by a function generator (Model 395, Wavetek, San Diego, CA, USA) and amplifier (model 240L, ENI Inc, Rochester, NY, USA). The electrical power was measured with a power meter (model 438A, Hewlett Packard, Palo Alto, CA, USA) and dual directional coupler (model C173, Werlatone, Brewster, NY, USA). The transducer’s electrical impedance was matched to the output impedance of the amplifier (50Ω) by an external matching network (built in-house).
Measurements of the peak negative pressure amplitude were performed with a calibrated membrane hydrophone (spot diameter 0.5 mm, GEC-Marconi Research Center, Chelmsford, England) for the entire pressure amplitude range used in degassed and deionized water. The values reported here are in the brain after taking into account ultrasound attenuation through 10 mm of brain, assuming an attenuation coefficient of 5 Np/m/MHz (0.43 dB/cm/MHz) (Goss et al. 1978). The half intensity beam diameter and length of the focal spot measured in a water tank with a needle hydrophone (spot diameter 0.2 mm, Precision Acoustics, Dorchester, UK) were 2.3 and 14 mm, respectively.
Ten seconds before each sonication, a bolus of ultrasound contrast agent (Optison, GE Healthcare, Milwaukee, WI, USA) was injected intravenously through the ear vein at a dosage of 50 µl per kg of body weight. This dosage was selected as it in the range recommended for human use (0.5–5.0 ml; i.e., 7.1–71 µl/kg for a 70 kg adult). Dosages of two and five times this amount were tested in select experiments. This contrast agent consists of pre-formed bubbles (human serum albumin shells filled with a perfluorocarbon gas). Immediately following the contrast agent injection, a bolus of 2 ml saline was given to flush the agent from an access line that extended out of the magnetic resonance imaging scanner. A delay of at least five min between sonications allowed the bubbles to mostly clear from the circulation. In each animal, one parameter was varied from location to location. The order of these sonications was varied from animal to animal to avoid bias in the experiments. In particular, it avoided bias between the first and last sonications in each animal that could occur due to the cumulative effects of ultrasound contrast agent that remained after the five min delay.
Experimental apparatus
The transducer was attached to a three axis manual positioning system and submerged in a tank of degassed, deionized water. The animal was positioned supine on a plastic plate that was placed above this tank. The ultrasound beam propagated vertically out of the tank through a hole in this plate. This hole was the opening of a thin plastic bag filled with degassed water that provided acoustic coupling between the water tank and the animal. The experiments were performed in a clinical 1.5T magnetic resonance imaging (MRI) unit (GE Healthcare, Milwaukee, WI, USA). A receive-only surface coil (7.6 mm diameter, GE Healthcare, Milwaukee, WI, USA) was placed below the head to provide imaging with a high signal-to-noise ratio. The ultrasound beam propagated through the hole in the coil.
MRI
T2-weighted fast spin echo images were used to select the target locations and to delineate the craniotomy to ensure the ultrasound beam was not clipped by the skull (parameters: repetition time/echo time (TR/TE): 2000/85 ms; echo train length (ETL): 8; matrix size: 256×256; field of view (FOV): 10 cm; slice thickness: 1.5 mm; number of excitations (NEX): 2). BBB disruption was detected in contrast-enhanced T1-weighted fast spin echo images (parameters: TR/TE: 500/15–23 ms; ETL: 4; BW: 16 kHz; matrix size: 256×256; NEX: 4; FOV: 10 cm; slice thickness: 1.5 mm; interslice spacing: 1.5 mm). T1-weighted imaging was acquired immediately after the last sonication in axial (horizontal) slices (perpendicular to the direction of the ultrasound beam propagation). This imaging was repeated multiple times after contrast injection (Magnevist®, Berlex Laboratories, Inc., Wayne, NJ, USA administered intravenously at a dose of 0.125 mmol per kg of body weight as a bolus injection), and then images in other orientations were acquired.
Histology
The animals were sacrificed 4h after the last sonication. Representative examples from each experimental group were selected for histological examination. The representative samples selected for histological analysis had a signal enhancement in contrast MRI that was within one standard deviation of mean enhancement for all the sonications applied at the nominal values described in the following section. The brains were fixed via transcardial perfusion (0.9%NaCl-250 ml, 10% buffered formalin phosphate –500 ml) followed by immersion fixation (10% buffered formalin phosphate). These brains were then embedded in paraffin and serially sectioned at 5 micrometers. Every 50th section (every 250 µm) was stained with hematoxylin and eosin (H&E) for light microscopy evaluation. For each targeted location, the section that showed the largest tissue effects was identified. One author (NV) who was blind to the acoustic parameters used performed the histology examination. This author did know the locations of the sonications.
Experimental groups
In designing these experiments, the sonication parameters were based on values found earlier that consistently produced BBB disruption with negligible effects to the brain at an ultrasound frequency of 690 kHz (Hynynen et al. 2005): pressure amplitude of 0.5 MPa, pulse repetition frequency (PRF) of 1 Hz, burst length of 10 ms, Optison dosage of 50 µl/kg. For each experimental group, one parameter was changed (burst length, PRF, or Optison dosage), and the other parameters fixed to these nominal values. In the case of the burst length, a range of acoustic pressure amplitudes were tested in additional experiments in order to investigate the threshold for BBB disruption for 0.1 and 1 ms burst lengths. These additional experiments were performed for the burst length experiments because the signal intensity enhancement was found to differ significantly from the other groups for a pressure amplitude of 0.5 MPa (see Results). Data from a previous study performed under identical conditions (Hynynen et al. 2005) was used along with data acquired here at 0.5 MPa to calculate the BBB disruption threshold for the 10 ms burst length. The parameters tested for experimental groups are listed in Table 1. Based on previous work in the brain (Hynynen et al. 1997; Vykhodtseva et al. 2000; Hynynen et al. 2001), it is known that the exposure levels used in this work produce no measurable effects to the brain tissue, so control locations without the contrast agent were not performed.
Table 1.
Sonication parameters and Optison dosages used in this study. All sonications were 20 s in duration.
| Experimental Group | Acoustic Power (W) | Pressure amplitude (MPa) | Ultrasound Frequency (MHz) | Burst (ms) | PRF (Hz) | Optison (µl/kg) | Locations with BBB disruption | Mean signal enhancement in MRI |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 0.4 | 0.69 | 0.1 | 1 | 50 | 20% (1/5) | 0.8 ± 2.0% |
| 0.2 | 0.5 | 0.69 | 0.1 | 1 | 50 | 17% (1/6) | 3.2 ± 4.2% | |
| 0.4 | 0.8 | 0.69 | 0.1 | 1 | 50 | 60% (3/5) | 7.2 ± 4.9% | |
| 0.8 | 1.1 | 0.69 | 0.1 | 1 | 50 | 100% (4/4) | 12.6 ± 2.3% | |
| 1.6 | 1.5 | 0.69 | 0.1 | 1 | 50 | 100% (4/4) | 13.1 ± 3.1% | |
| 2 | 0.1 | 0.4 | 0.69 | 1 | 1 | 50 | 17% (1/6) | 0.4 ± 2.1% |
| 0.2 | 0.5 | 0.69 | 1 | 1 | 50 | 67% (4/6) | 3.0 ± 1.0% | |
| 0.4 | 0.8 | 0.69 | 1 | 1 | 50 | 100% (4/4) | 8.9 ± 3.3% | |
| 0.8 | 1.1 | 0.69 | 1 | 1 | 50 | 100% (4/4) | 10.9 ± 1.4% | |
| 3 | 0.2 | 0.5 | 0.69 | 10 | 1 | 50 | 100% (11/11) | 12.4 ± 4.6% |
| 0.2 | 0.5 | 0.69 | 10 | 0.5 | 50 | 88% (7/8) | 8.7 ± 3.6% | |
| 0.2 | 0.5 | 0.69 | 10 | 2 | 50 | 100% (8/8) | 11.1 ± 3.0% | |
| 0.2 | 0.5 | 0.69 | 10 | 5 | 50 | 89% (8/9) | 9.1 ± 4.7% | |
| 4 | 0.2 | 0.5 | 0.69 | 10 | 1 | 100 | 100% (10/10) | 12.6 ± 4.1% |
| 0.2 | 0.5 | 0.69 | 10 | 1 | 250 | 100% (10/10) | 14.8 ± 6.8% | |
Data Analysis
The signal intensity enhancement was found by calculating the percent increase in image intensity in a 3×3 voxel region of interest (ROI) at the sonication target in the contrast-enhanced T1-weighted imaging. The percent increase in a 5×5 voxel ROI in a nearby non-sonicated region (control) was subtracted from this value to exclude enhancement due to contrast in the vasculature. The average signal intensity in the four or five images acquired between 10 and 20 minutes after the contrast injection was used in the calculations. BBB disruption was determined to have occurred if the mean percent change in the target ROI was greater than the standard deviation of the larger control ROI.
For the experiments that investigated burst length, the probability for BBB disruption, with 95% confidence intervals, was estimated as a function of pressure amplitude using probit analysis, a regression model for the analysis of categorical data often used for bioassay work (Finney 1971). The threshold was defined as the value where the probability for BBB disruption was estimated to be 50%. All data analysis was performed by one author (NM) using software written in Matlab (version 6.1, Mathworks, Natick, MA, USA).
Results
General MRI and Histology Observations
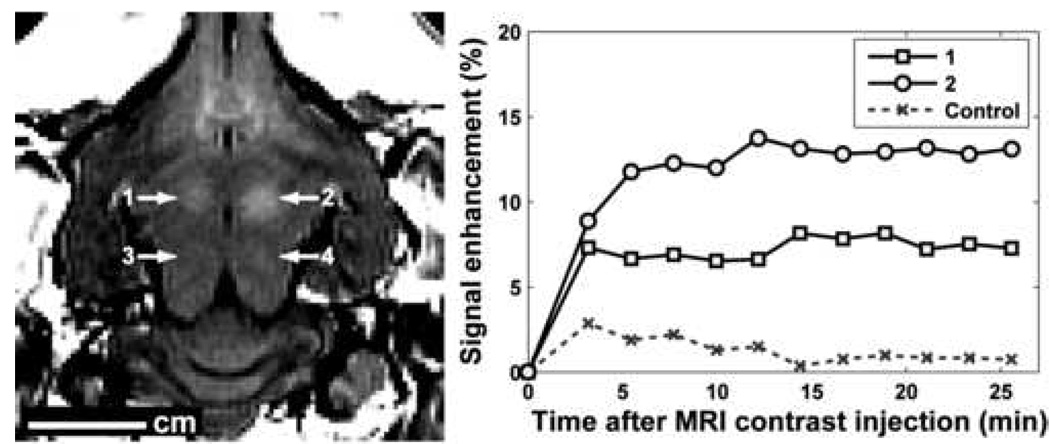
BBB disruption was observed in contrast-enhanced MRI as localized regions of signal enhancement at the sonication target (Figure 1). As multiple images were acquired after contrast injection, the magnitude of the enhancement at the focal coordinate increased initially and was then constant after a few minutes over the time examined.
Figure 1.
Left: MR image showing signal enhancement due to localized BBB disruption at two locations in the rabbit brain. This brain was sonicated at four locations with a burst length of 1 ms. Locations 1–4 were sonicated at 0.8, 1.1, 0.8 and 0.5 MPa, respectively. BBB disruption was not observed for locations 3 and 4. An axial (horizontal) slice is shown that was perpendicular to the direction of the ultrasound beam in the focal plane. Right: signal enhancement as a function of time after the injection of MRI contrast agent for the two locations with BBB disruption and a control (non-sonicated) location. This injection occurred 31, 7, 19, and 13 min after the sonications at locations 1–4 respectively.
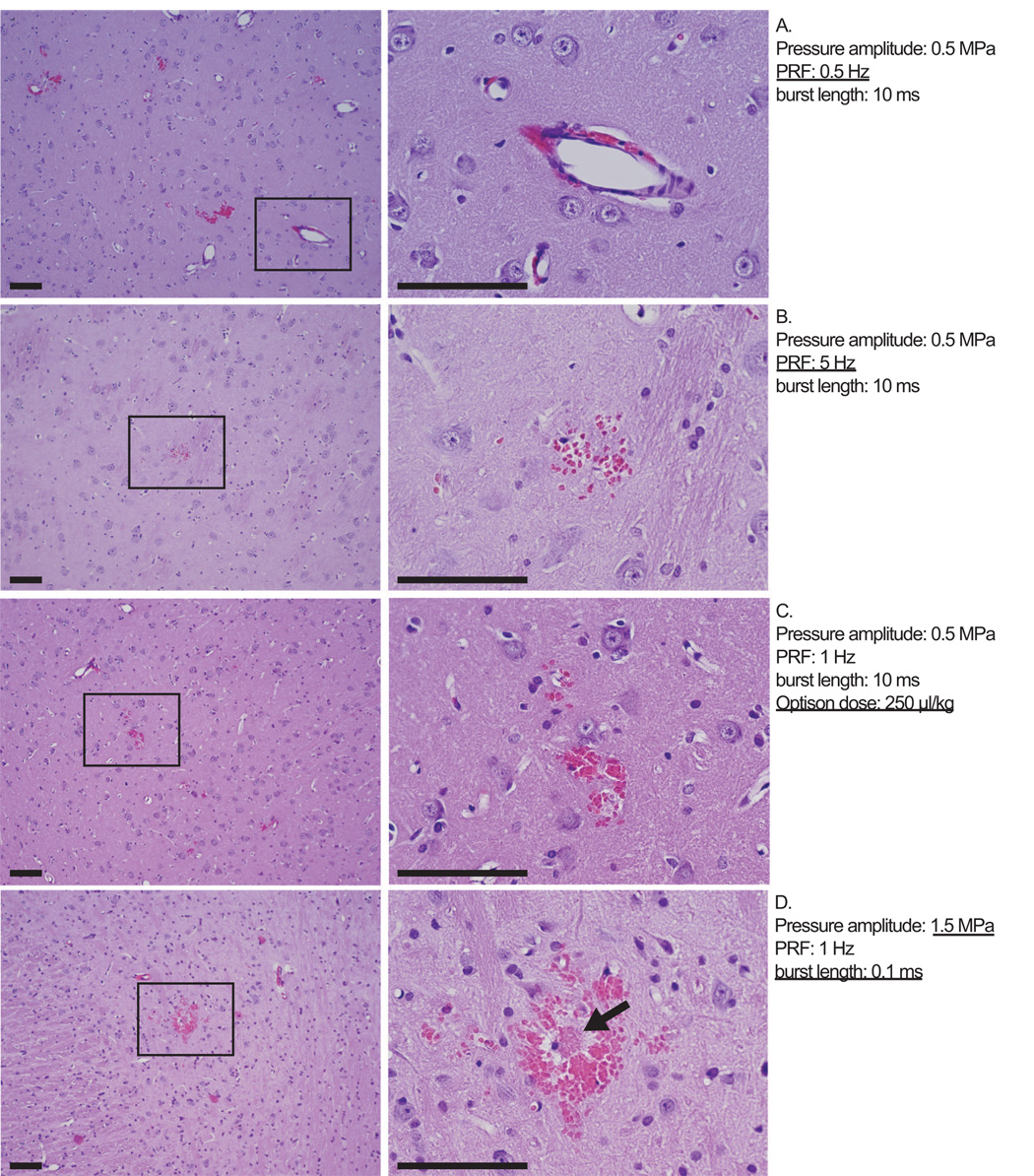
Histological changes were small and generally limited to minor vascular damage, as indicated by extravasated erythrocytes that were found in the sonicated regions. These extravasations ranged from one or a few isolated red blood cells to clusters of cells less than approximately 150 µm in diameter. Such extravasations were found in 59 of the 63 locations examined. In 26 of these 59 locations, the total number of clusters of extravasations found in the entire sonicated area in the section with the largest effect was less than five. The brain parenchyma in these locations appeared to be otherwise mostly unaffected except for four of the sonicated locations where tiny damaged regions (less than 100 µm in diameter) were found. For the 63 representative examples examined, which all had similar amounts of MRI contrast enhancement, no clear difference could be found between locations sonicated with different parameters. Examples of the histology are shown in Figure 2.
Figure 2.
Microphotographs of H&E sections for four sonicated locations with similar MRI contrast enhancement. Despite changing the acoustic parameters or dose of ultrasound contrast agent, the tissue effects were similar: minor vasculature damage as indicated by a one or a few red blood cells scattered about or small (diameter less than approximately 150 µm) clusters of erythrocytes, as shown in these four examples. There brain tissue appeared to be generally unaffected, except in four cases overall where tiny (less than 100 µm) damaged regions were found associated with the extravasations. An example of such damage is indicated by an arrow in (D). The parameters used are indicated; those deviating from nominal values are underlined. Bar: 100 µm.
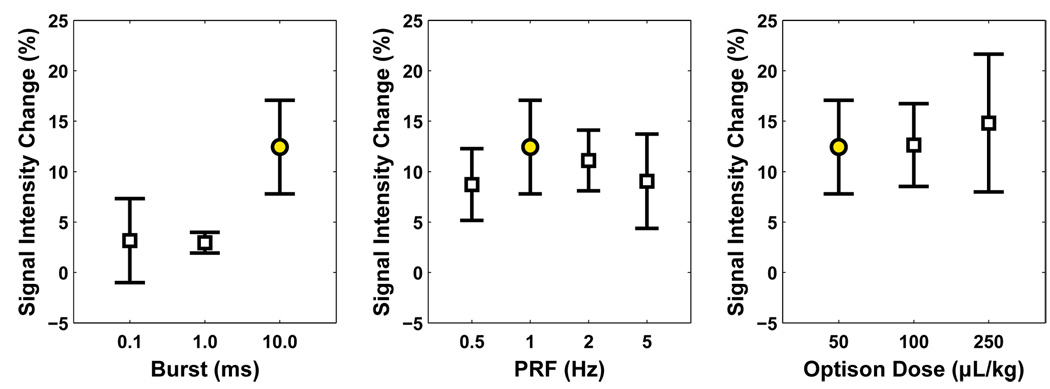
Effect of burst length, pulse repetition frequency, and Optison dosage
The mean MRI signal intensity enhancement (±SD) is plotted as a function of burst length, pulse repetition frequency, and Optison dosage in Figure 3 for the sonications performed at a peak negative pressure amplitude of 0.5 MPa. The data is also listed in Table 1. No significant difference in enhancement was observed for the different pulse repetition frequencies or for the Optison dosages tested. However, one location in each of the groups that tested pulse repetition frequencies of 0.5 and 5 MHz did not have BBB disruption.
Figure 3.
Mean signal intensity enhancement (± SD) measured in contrast-enhanced MRI for sonications performed where the burst length (left), pulse repetition frequency (center), and dose of ultrasound contrast agent (right) were varied. Only sonications with burst lengths of 0.1 or 1.0 ms showed a significant difference from the sonications with the nominal values (a 10 ms burst length, a 1 Hz pulse repetition frequency, and an Optison dosage of 50 µL/kg). The data from these sonications (indicated by filled circles) is repeated in all three graphs. All sonications in these graphs were applied with a peak negative pressure amplitude of 0.5 MPa (estimate in brain).
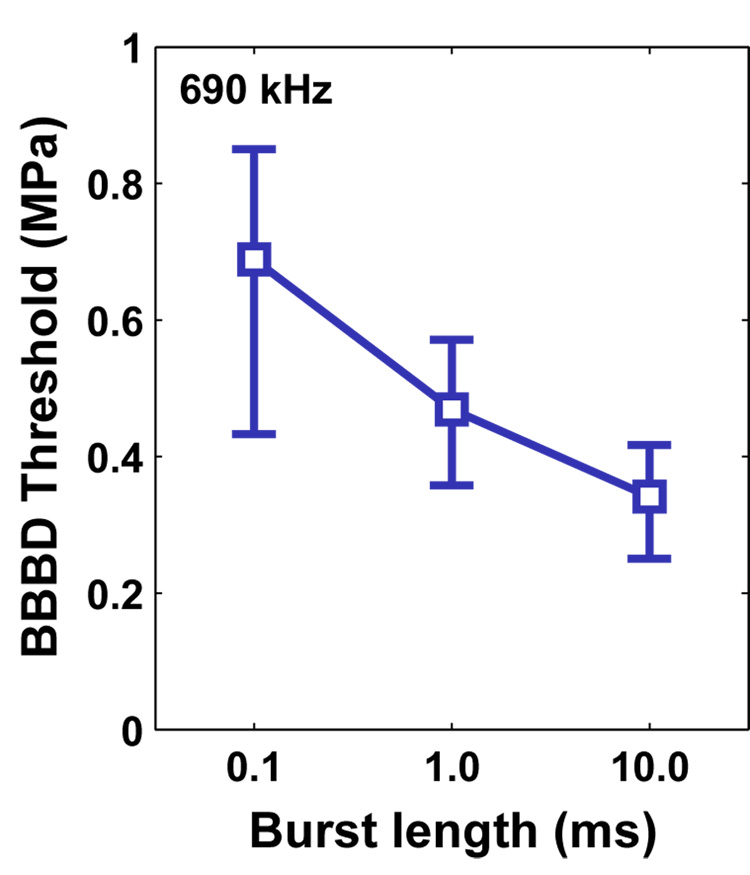
However, sonications at a pressure amplitude of 0.5 MPa with a 10 ms burst produced significantly more (P<0.01) enhancement than those with burst lengths of 1 or 0.1 ms. The BBB disruption threshold, defined as the peak negative pressure amplitude where the probability for disruption was estimated to be 50% based on the probit regression, also decreased as a function of the increasing burst length (Figure 4). The thresholds were estimated to be 0.69 (CI: 0.53 – 0.95), 0.47 (CI: 0.41 – 0.52), and 0.36 (CI: 0.29 – 0.44) MPa, respectively, for 0.1, 1, and 10 ms bursts.
Figure 4.
Plot showing the threshold for BBB disruption (with 95% confidence intervals) as a function of the burst length. This threshold was defined as the pressure amplitude (estimate in brain) where the probability for BBB disruption was estimated to be 50% using probit regression.
Discussion
Of the parameters tested, only the burst length had a significant effect on the magnitude of the BBB disruption as determined by the percent increase in signal enhancement measured in contrast-enhanced T1-weighted MRI. Thus, for the range of parameters tested, this work suggests that the magnitude of BBB disruption is insensitive to the number of pulses delivered and the number of microbubbles present in the vasculature. The data also suggests that these parameters as well the burst length do not change the histological effects associated with the disruption, at least for sonications where the magnitude of the BBB disruption is similar. While the reasons for these results are not obvious, one can speculate on their relationship to potential mechanisms of the BBB disruption.
Previous studies investigating suggest that the mechanism for the BBB disruption may be partially related to a physiological response to the sonication instead of a direct physical effect. Evidence for such a response has been seen in electron microscopy, where in addition to tracer being found in junctions between endothelial cells, it was found in vesicles that appeared to be transporting the agent to the parenchyma (Sheikov et al. 2004; Hynynen et al. 2005; Hynynen et al. 2006). Also, in vivo multiphoton microscopy of the brain vasculature during sonication suggests that the pulses were associated with temporary constriction of the blood vessels followed immediately by extravasation of fluorescent tracer into the parenchyma; after a period of time (seconds to minutes) the vessels returned to their normal state (Raymond et al. 2006). If the BBB disruption is largely a physiological response, for example related to this temporary vessel constriction, perhaps adding more pulses per second did not produce more disruption because of the time needed for the vessels to return to normal from their constricted state. Similarly, if the effect of sonication on a bubble causes a certain length of blood vessel to constrict, perhaps adding more bubbles per vessel length did not change the outcome.
Any physiological mechanism triggered by sonication are likely related to interactions between the bubbles and the vessel walls, which include oscillatory forces, radiation force along the direction of the ultrasound field, and shear stress related to streaming of fluid around the bubbles. For short pulses, the results of each of these interactions will depend on the burst length: the bubble radius during oscillation, the displacement induced by radiation force and the velocity of the fluid during streaming. If the burst length is increased, these things will saturate and a longer pulse may not increase the effect. This may explain why in previous work 10 ms bursts produced similar extravasation of MRI contrast agent as 100 ms bursts for the same acoustic power level (Hynynen et al. 2001). If the burst is decreased, the interaction may not have time to develop and one may not expect to find BBB disruption at all for a fixed power level. This may explain why previous tests using sonications with very short bursts (10 µs, 2 kHz PRF) failed to produce BBB disruption below power levels that resulted in tissue damage (Hynynen et al. 2003).
In addition to physiological changes induced by sonication, the magnitude of the BBB disruption will certainly also be related to any vascular damage that is produced during sonication, which is associated with red blood cell extravasation and is probably related to inertial cavitation (McDannold et al. 2006). If a large amount of such vessel damage is produced due to inertial cavitation, it may be the dominant source of leakage of tracer into the brain. In this case, we may expect the magnitude of the disruption to be correlated with the PRF and the dosage of ultrasound contrast agent as well as the burst length, since they all will affect either the inertial cavitation threshold or the number of sites where inertial cavitation occurs. In this study, only a very small number of regions containing extravasations were found, so perhaps vessel damage was not the dominant mechanism. In a separate study in rats (Treat et al. 2007), the amount of a chemotherapy agent (liposomal doxorubicin) delivered to the brain was strongly correlated to the dosage of ultrasound contrast agent. Perhaps in that study, micro-damage to the vessel walls was a significant source of the agent’s delivery. Indeed, sonication using higher dosages of ultrasound contrast agent, which was associated with a large increase in the drug delivery, was also associated with vascular damage and associated damage to the brain parenchyma. Similarly, in a recent study, delivery of Evan’s Blue to the rat brain increased as a function of ultrasound contrast agent dose (Yang et al. 2007). In that study, higher doses were also associated with tissue damage.
The above discussion is speculative and is based on clues from other studies that used different ultrasound frequencies or other acoustic parameters. An alternative explanation for our results could be simply that the variation in the magnitude BBB disruption from location-to-location was too large to detect the effect of varying the different parameters. Future work testing a wider range of parameters may show dependencies that were undetected here. If one devises a means to measure something that is related to the interaction of the ultrasound and the tissue during sonications (instead of using just the input values), one may also be able to reduce the amount of variation in the disruption and subtle parametric dependencies may be elucidated. Some of the variation may have been due to different amounts of time between the four sonications that were performed in each brain and the injection of the MRI contrast agent or differing amounts of residual ultrasound contrast agent that remained from previous sonications.
Future work should investigate other parameters that were not tested here, such as ultrasound frequency and sonication duration. One should also investigate how different parameters affect the length of time that the BBB is disrupted and the type of damage that is produced for each parameter for exposure levels above the threshold for BBB disruption. We also only performed limited histological examination of the brains and only examined acute effects. Previous studies using different ultrasound parameters have not found apoptotic or ischemic regions associated with the sonications, as one may expect if serious vasculature damage was produced (Hynynen et al. 2005; McDannold et al. 2005; Hynynen et al. 2006). Those studies also examined the brains up to one month after sonication. Since the histological changes in the present study were the same as in those previous works, one may expect a similar outcome for the parameters tested here. Finally, one of the locations in each of the groups that investigated pulse repetition frequencies of 0.5 and 5 Hz did not have BBB disruption after sonication. We assumed that this was the result of normal experimental variation. It could indicate, however, that the BBB disruption threshold is higher for these two parameters. Future work will be necessary to determine if that is true.
Conclusions
This data suggests that for the range of parameters tested, the magnitude of the BBB disruption induced by ultrasound pulses does not depend on the pulse repetition frequency or the dosage of ultrasound contrast agent. However, the magnitude of the disruption decreases and the threshold for BBB disruption increases as the burst length is shortened. The data also suggests that none of the parameters that were varied will produce different histological changes in the brain, at least for sonications that produced similar amounts of BBB disruption.
Acknowledgements
Support: NIH (R01EB003268, R33EB000705, U41RR019703). The authors thank Yongzhi Zhang for his help with these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Pernot M, Small SA, Konofagou EE. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med Biol. 2007;33:95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, McAllister LD, Bubalo JS, Kraemer DF, Fortin D, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–647. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit Analysis. Cambridge, U.K.: Cambridge University Press; 1971. [Google Scholar]
- Goss SA, Johnston RL, Dunn F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J Acoust Soc Am. 1978;64:423–457. doi: 10.1121/1.382016. [DOI] [PubMed] [Google Scholar]
- Guerin C, Olivi A, Weingart JD, Lawson HC, Brem H. Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest New Drugs. 2004;22:27–37. doi: 10.1023/b:drug.0000006172.65135.3e. [DOI] [PubMed] [Google Scholar]
- Hynynen K, Clement GT, McDannold N, Vykhodtseva N, King R, White PJ, Vitek S, Jolesz FA. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: A preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52:100–107. doi: 10.1002/mrm.20118. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Martin H, Jolesz FA, Vykhodtseva N. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (Optison®) Ultrasound Med Biol. 2003;29:473–481. doi: 10.1016/s0301-5629(02)00741-x. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood–brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurgery. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- Hynynen K, Vykhodtseva NI, Chung AH, Sorrentino V, Colucci V, Jolesz FA. Thermal effects of focused ultrasound on the brain: determination with MR imaging. Radiology. 1997;204:247–253. doi: 10.1148/radiology.204.1.9205255. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: A feasibility study. Ultrasound Med Biol. 2007;33:584–590. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Maravilla KR, Frenkel EP, Rapaport SI, Hill SA, Barnett PA. Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979;64:684–688. doi: 10.1172/JCI109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- Raymond SB, Skoch J, Hynynen K, Bacskai BJ. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600336. [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- Vykhodtseva NI, Sorrentino V, Jolesz FA, Bronson RT, Hynynen K. MRI detection of the thermal effects of focused ultrasound on the brain. Ultrasound Med Biol. 2000;26:871–880. doi: 10.1016/s0301-5629(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Yang FY, Fu WM, Yang RS, Liou HC, Kang KH, Lin WL. Quantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrier disruption. Ultrasound Med Biol. 2007;33:1421–1427. doi: 10.1016/j.ultrasmedbio.2007.04.006. [DOI] [PubMed] [Google Scholar]