Structured Abstract
Objectives
We examined whether the presence and extent of late gadolinium enhancement (LGE) by CMR predict adverse outcomes in nonischemic cardiomyopathy (NICM) patients.
Background
Morbidity and mortality is high in NICM patients. However, the clinical course of an individual patient is unpredictable and current risk stratification approaches are limited. Cardiovascular magnetic resonance (CMR) detects myocardial fibrosis, which appears as LGE after contrast administration and may convey prognostic importance.
Methods
In a prospective cohort study, 65 NICM patients with LVEF ≤35% underwent CMR before placement of an internal cardioverter defibrillator (ICD) for primary prevention of sudden cardiac death. CMRs were analyzed for the presence and extent of LGE, and for LV function, volumes, and mass. Patients were followed for an index composite endpoint of three cardiac events: hospitalization for heart failure, appropriate ICD firing, and cardiac death.
Results
42% (n=27) of patients had CMR LGE, averaging 10±13% of LV mass. During a 17 month median follow-up, 44% (n=12) of patients with LGE had an index composite outcome event, versus only 8% (n=3) of those without LGE (p<0.001 for Kaplan-Meier survival curves). After adjustment for LV volume index and functional class, patients with LGE had an eight-fold higher risk of experiencing the primary outcome (hazard ratio 8.2, 95% CI 2.2–30.9, p=0.002).
Conclusions
CMR LGE in NICM patients strongly predicts adverse cardiac outcomes. CMR LGE may represent the end-organ consequences of sustained adrenergic activation and adverse LV remodeling, and its identification may significantly improve risk stratification strategies in this high risk population.
Condensed Abstract
Predicting prognosis in nonischemic cardiomyopathy patients is challenging and current risk stratification approaches are limited. Cardiovascular magnetic resonance (CMR) detects myocardial fibrosis, which appears as late gadolinium enhancement (LGE). The presence of LGE predicts an eight-fold increased risk of an adverse cardiac outcome (HR 8.1, 95% CI 1.9–33.7, p=0.004), after controlling for baseline variables. CMR LGE may reflect the transition from compensated to decompensated state resulting from long-term stressors such as sustained adrenergic activation and/or the mechanical disadvantages caused by LV remodeling leading to increasing fibrosis. Identifying CMR LGE may significantly improve risk stratification strategies in this high risk population.
Keywords: cardiomyopathy, prognosis, magnetic resonance imaging
Introduction
Patients with nonischemic cardiomyopathy (NICM) comprise one-third of the heart failure (HF) population and are at risk for significant morbidity and mortality (1,2). NICM is the most common indication for heart transplantation. Ten-year survival is below 60% (3) with deaths often preceded by frequent hospitalizations for HF exacerbations (4). Forty percent of deaths are sudden cardiac death (SCD) and placement of an ICD for primary prevention reduces arrhythmic deaths in NICM patients with left ventricular ejection fraction (LVEF)≤35% (5–7). However, risk stratification remains challenging, particularly in the individual patient in whom the clinical course frequently correlates poorly with LVEF. Better risk-stratification tools might allow earlier intervention in high-risk patients, improving both quality of life and survival.
In patients with NICM, myocardial fibrosis has been identified pathologically (8). Macroscopic regions of fibrosis have also been visualized by cardiovascular magnetic resonance (CMR), appearing as areas of late gadolinium enhancement (LGE) (9–11). Increasing amounts of fibrosis potentially result in increased LV stiffness and reduced LV compliance, thereby progressively impairing both diastolic and systolic function, reducing cardiac output (8). Myocardial fibrosis may also form a substrate for lethal reentrant ventricular arrhythymias (12,13). We thus hypothesized that the presence and extent of CMR LGE are associated with a higher risk of adverse cardiac outcomes in patients with NICM and LVEF ≤35%.
Methods
Patients
We conducted this pre-specified, single-center prospective cohort study between April 2004 and April 2007, as part of a larger prospective registry of both nonischemic and ischemic cardiomyopathy patients undergoing ICD implantation for primary prevention of SCD. The present study included consecutive, non-selected patients with NICM and LVEF ≤35% by a clinically indicated non-CMR study (echocardiography or nuclear), referred for ICD by their treating cardiologist. All patients had coronary angiography and were classified as nonischemic if they had no history of MI or revascularization and no evidence of coronary artery stenoses >50% of 2 or more epicardial vessels or left main or proximal left anterior descending coronary artery stenosis >50% (14). We excluded patients with prior arrhythmic indications for ICD placement (such as a history of syncope, cardiac arrest, or ventricular arrhythmias); New York Heart Association (NYHA) functional class IV; and acute myocarditis, congenital heart disease, hypertrophic cardiomyopathy, or infiltrative heart disease. Renal insufficiency with creatinine clearance <30 ml/min was added as an exclusion in July 2006. The study protocol was approved by the Johns Hopkins Hospital Institutional Review Board. All patients gave written informed consent.
MRI protocol
Prior to ICD implantation, patients underwent CMR using a 1.5 Tesla scanner (Signa CV/i, GE Healthcare Technologies, Waukesha, WI or Siemens Avanto, Erlangen, Germany). Cine images were acquired with a steady-state free precession pulse sequence (TR 2.5–3.8, TE 1.1–1.6, average in-plane resolution 1.5×2.4 mm, flip angle 45–60º, temporal resolution 40–45 msec) in long-axis planes and contiguous 8-mm short-axis slices from the mitral annulus to the apex. Fifteen to thirty minutes after intravenous administration of 0.2 mmol/kg gadodiamide (Omniscan, GE Healthcare Technologies, Waukesha, WI), delayed contrast-enhanced images were acquired using inversion-recovery fast gradient-echo pulse sequences (15,16) in the same short-axis locations as the cine images. In order to exclude artifact, short axis imaging was repeated in 2 different phase-encoding directions and cross-sectional long axis views were also acquired. Imaging parameters were: TR 5.4–8.3 ms, TE 1.3–3.9 ms, average in-plane spatial resolution 1.4–1.5×2.2–2.4 mm, 8-mm slice thickness, 2-mm gap, TI 175–300 msec (adjusted as needed in the delayed enhancement image acquisitions to optimally null the signal of normal myocardium), 1–2 R-R imaging, flip angle 20–25°.
Data Analysis
Analysis of the CMR DICOM images was performed using CINEtool software (GE Healthcare Technologies, Waukesha, WI). LVEF, volumes and mass were quantified from the cine images by standard methods. LV volumes and mass were normalized to body surface area. Two observers blinded to the clinical outcome independently determined the dichotomous presence or absence of LGE by reviewing all short and long axis contrast-enhanced images; regions of elevated signal intensity had to be confirmed in 2 spatial orientations.
If CMR LGE was present, the quantitative extent of hyperenhancement was defined as regions with abnormally increased signal intensity > peak remote (17) For each short axis cross-section, after the endocardial and epicardial borders were traced, a region of interest (ROI) averaging 50 mm2 was defined within the normal, remote myocardium in an area with uniform myocardial suppression free of artifacts. The peak signal intensity (SI) within the remote ROI was then determined. Total myocardial LGE was defined as abnormal myocardium with SI above peak remote SI and expressed as a % of total LV mass and as a total volume.
Intraobserver reproducibility of the extent of LGE using the peak remote threshold was measured. In addition, comparison was made to the extent of the LGE region quantified using a threshold of >2 standard deviations above mean remote SI (11).
Clinical Follow-up
Patients were followed at 3–6 month intervals after ICD placement via clinic visits or phone calls. The primary outcome of the study was the index combined endpoint of cardiac death (both sudden and non-sudden), appropriate ICD firing and hospitalization for decompensated HF requiring intravenous diuretics with or without inotropes. No patient was lost to follow-up. Events were adjudicated by an independent committee blinded to the CMR results. Deaths were classified according to the modified Hinkle-Thaler system (18). Appropriate ICD firing was defined as a shock for sustained ventricular tachycardia above the programmed rate cutoff of the ICD (generally >180 bpm) or ventricular fibrillation.
Statistical Analysis
Continuous variables are expressed as mean±SD. The Wilcoxon rank-sum test was used to compare continuous baseline and CMR characteristics of the patients grouped by the dichotomous presence or absence of CMR LGE. Fisher’s exact test was used for categorical variables. Kaplan-Meier curves and the Wilcoxon test of Breslow were used to compare univariate survivor functions (time to index composite event). Cox proportional-hazards models were developed. We included as potential co-variates, age, gender, baseline NYHA functional class, LVEF, mass and volume indices, which are known from prior research to affect prognosis in NICM (19). No other additional baseline characteristics were found to influence the relationship between CMR LGE and outcome. Since there was strong collinearity (correlation coefficient>0.6) between LVEF, LV volumes and mass, only LVEDV was included in the models. Forward stepwise regression (p<0.10 for entry, p>0.05 for removal) was used to arrive at a parsimonious model (Model 1). Given the well-established prognostic importance of LVEDV, this was added to the parsimonious model for the final analysis of all patients (Model 2). Followup duration was measured from the CMR study date. Bland-Altman repeatability analysis was used to compare quantification of the LGE region using the thresholds of peak remote versus 2 SD above mean remote as well as intraobserver variability. A two-tailed p-value<0.05 defined statistical significance.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline characteristics (Table 1)
Table 1.
Baseline demographic, clinical and MRI characteristics of the patients, grouped by absence or presence of CMR late gadolinium enhancement (LGE).
| Variable | LGE ABSENT BY CMR (N=38) | LGE PRESENT BY CMR (N=27) | p-value |
|---|---|---|---|
| Age - yrs | 55±12 | 56±9 | P=0.99 |
| Male gender – no. (%) | 19 (50) | 23 (85) | P=0.003 |
| Caucasian – no. (%) | 19 (50) | 15 (58) | P=0.14 |
| Duration of cardiomyopathy diagnosis (years) | 4.0±4.1 | 4.1±4.4 | P=0.74 |
| Entry EF (non-MRI method) (%) | 21±9 | 19±6 | P=0.47 |
| Single vessel CAD >50% (non-left main or proximal LAD vessels) | 2 (5) | 3 (11) | P=0.38 |
| NYHA functional class | P=0.52 | ||
| Class I | 7 (18) | 3 (11) | |
| Class II | 16 (42) | 15 (56) | |
| Class III | 15 (39) | 9 (33) | |
| Medication use – no.(%) | |||
| ACEI or ARB | 33 (87) | 25 (92) | P=0.46 |
| Beta-blocker | 36 (95) | 24 (89) | P=0.64 |
| Diuretic | 22 (65) | 19 (73) | P=0.38 |
| Spironolactone | 11 (29) | 8 (30) | P=0.95 |
| Digoxin | 15 (39) | 5 (19) | P=0.07 |
| Antiarrhythmics (amiodarone) | 2 (5) | 4 (15) | P=0.19 |
| Lipid-lowering | 16 (42) | 16 (59) | P=0.17 |
| Received biventricular ICD (%) | 15 (41) | 8 (30) | P=0.37 |
| CMR measurements | |||
| LVEF (%) | 26±9 | 22±10 | P=0.20 |
| LVEDV index (ml/m2) (all patients) | 126±43 | 149±64 | P=0.10 |
| Female | 120±37 | 137±96 | |
| Male | 132±49 | 151±67 | |
| LVESV index (ml/m2) (all patients) | 96±41 | 120±66 | P=0.08 |
| Female | 89±36 | 109±44 | |
| Male | 104±50 | 122±69 | |
| LV mass (grams/m2) (all patients) | 78±24 | 90±43 | P=0.16 |
| Female | 73±17 | 71±21 | |
| Male | 83±29 | 93±45 | |
| Extent of LGE (mean±SD) | -- | 10±13% of LV mass | -- |
| Median extent | 5% | ||
| Interquartile range | 3–16% | ||
| Total volume (ml) | 17±21 ml |
We enrolled 65 patients whose baseline clinical characteristics are presented in Table 1. Median follow-up for the survivors was 17 months. Twenty-seven patients (42%) had CMR LGE with the following regional patterns: septal mid-wall (Fig 1A, n=8), subendocardial extending to epicardial surface (Fig 1B, n=7) and patchy foci (Fig 1C, n=12) often including the RV-LV septal insertion points.
Figure 1. CMR late gadolinium enhancement patterns seen in the study group.
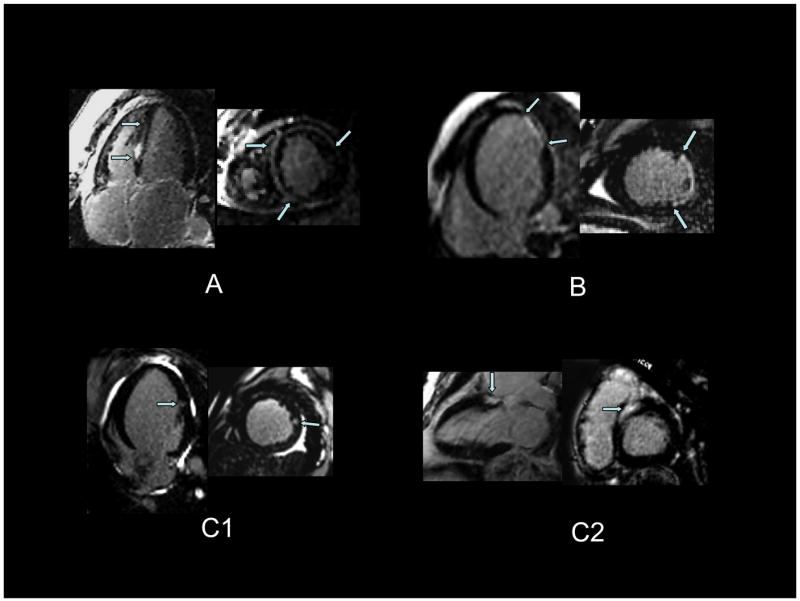
Panel A shows predominantly mid-wall enhancement involving the septal, anterior, and anterolateral walls. Panel B shows apical-lateral near-transmural enhancement (subendocardial to epicardial enhancement). Panel C shows patterns of patchy foci not following an epicardial coronary perfusion territory. In panel C1, there is a focus of mid-lateral wall enhancement. In panel C2, there is basal septal enhancement.
Patients with and without CMR LGE had similar baseline characteristics, including duration of cardiomyopathy diagnosis, entry NYHA functional class, medication usage (both at baseline and at follow-up), and bi-ventricular ICD usage, although LGE was more frequent in men. Only 5 of the 65 patients had single vessel CAD (not involving the left main or proximal LAD). This is consistent with cardiomyopathy “out of proportion” to CAD extent and none of the 5 had evidence of LGE in that specific coronary distribution. LVEF trended lower and LV volume and mass indices were higher in those patients with LGE compared to those without LGE.
Serum sodium and creatinine values were identical in both groups. Pro-BNP values (pg/ml) were obtained in a subset of patients (n=42) and were significantly different between the 2 groups: 1041±1448 for patients without LGE (n=24) vs 4497±6519 for those with LGE (n=18), p=0.002.
Reproducibility of CMR LGE
For the dichotomous presence or absence of LGE, there was complete intra-observer agreement. There was only one case of inter-observer disagreement that was resolved by consensus with a third independent observer. For quantification of the LGE region, intra-observer variability using the peak remote threshold was extremely low with mean difference (bias) of 0.1 ml (95% CI −0.3–0.5); limits of agreement, −1.9–2.1 ml. Low interobserver variability of this method has previously been shown (17). Compared with the > 2 SD above mean remote SI, the current methodology for quantifying LGE resulted in slightly larger regions [17.4±20.8 versus 16.6±21.2 ml, bias 0.8, 95% CI 0.3–1.3, limits of agreement, −1.6–3.2].
CMR LGE and clinical outcome
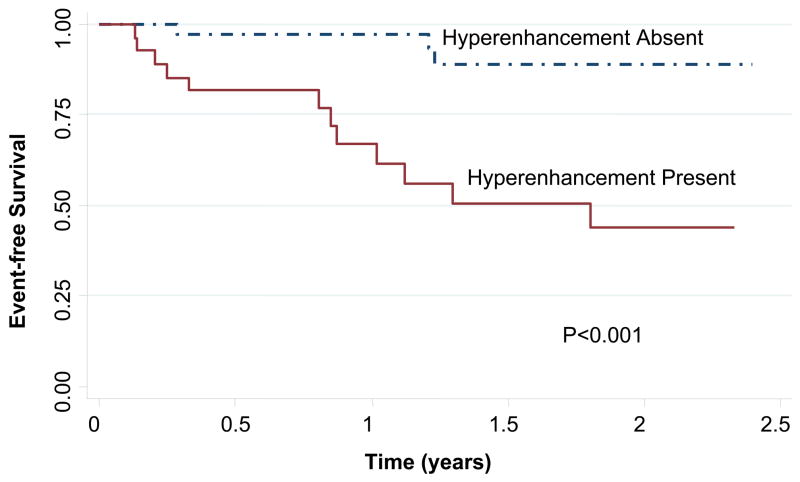
The presence of CMR LGE was associated with an increased risk of sustaining an index composite cardiac event over time. Kaplan-Meier curves (Figure 2) for the index composite endpoint showed a significant difference between patients with and without CMR LGE (Wilcoxon-Breslow p<0.001). Twelve patients (44%) with LGE had an index composite event (8 with a HF hospitalization and 4 with an appropriate ICD firing), compared to 3 patients (8%) without LGE, all of whom had an ICD firing (Table 2). The median time to an index event was 10.2 months. Among the 12 patients with LGE and index events, additional subsequent events were common (Table 2, “Cumulative number of events”): 3 patients died within 2 months of the index event (2 patients with an initial HF hospitalization subsequently died of LV pump failure and 1 patient with an initial ICD firing died of SCD); 2 patients had multiple appropriate ICD firings on separate dates; and 3 patients with an index HF hospitalization had subsequent HF hospitalizations. There were no deaths or HF hospitalizations among patients without CMR LGE.
Figure 2.
Kaplan-Meier event-free survival curve for the occurrence of an index composite event in patients, grouped by presence or absence of CMR late gadolinium enhancement. Wilcoxon-Breslow p<0.001 for the two survival curves.
Table 2.
Number of index and cumulative events grouped by presence or absence of CMR late gadolinium enhancement (LGE)
| Variable | LGE ABSENT BY CMR (N=38) | LGE PRESENT BY CMR (N=27) |
|---|---|---|
| Number of patients who experienced an index composite outcome* | 3 (8%) | 12 (44%) |
| HF hospitalization | 0 | 8 |
| Appropriate ICD firing | 3 | 4 |
| Cardiac death | 0 | 0 |
| Cumulative number of events over the follow-up period | 3 | 25 |
| HF hospitalization | 0 | 13 |
| Appropriate ICD firing | 3 | 9 |
| Cardiac death | 0 | 3 |
Note that the primary statistical analysis in the text was performed using the number of patients experiencing an index composite outcome and time to the index event. The cumulative number of events was not used in any statistical analysis.
By univariate analysis (Table 3, upper half), the presence of CMR LGE was strongly associated with cardiac events [hazard ratio (HR) 7.1 (95% CI, 2.0–25.3, p=0.002)]. This association was unchanged in multivariate analysis: CMR LGE had a hazard ratio of 8.2 (95% CI 2.2–30.9, p=0.002) for an index composite event after adjustment for HF class and LVEDV index. When the 5 patients with CAD were excluded, both univariate and multivariate results continued to show significant association between CMR LGE and adverse outcome (Table 3, lower half). Because LVEF, LV dimensions, LV volume and mass indices were correlated, we used only LV end-diastolic volume (LVEDV) index in Cox proportional hazard models; the results were unchanged when LVEF was substituted for LVEDV index or when all CMR volume and mass indices were used and when CHF class was modeled as a categorical rather than an ordinal variable. Similarly, the addition of serum sodium and creatinine values was not predictive of outcome and did not change the multivariate results. Including whether or not a biventricular pacemaker was placed also did not change the multivariate results, neither did medication usage. When the endpoint was limited to cardiac death and appropriate ICD firing, thus excluding HF hospitalization, LGE continued to predict a significantly worse outcome by Kaplan-Meier analysis (Wilcoxon-Breslow p=0.03): 22% (n=6) of patients with LGE reached this secondary endpoint versus only 8% (n=3) without LGE.
Table 3.
Cox proportional hazards analysis for the time to occurrence of an index composite cardiac event.
| Variable | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS MODEL 1* | MULTIVARIATE ANALYSIS MODEL 2† | |||
|---|---|---|---|---|---|---|
| UNADJUSTED HR VALUE (95% CI) | P- | ADJUSTED HR VALUE (95% CI) | P- | ADJUSTED HR VALUE (95%CI) | P- | |
| Age (5 year increments) | 1.1 (0.9–1.5) | 0.28 | --- | --- | --- | --- |
| Gender | 1.8 (0.5–6.2) | 0.39 | --- | --- | --- | --- |
| NYHA functional class | 2.0 (0.8–4.5) | 0.12 | 2.7 (1.0–7.2) | 0.04 | 2.6 (1.0–7.0) | 0.05 |
| CMR LVEDV index (ml/m2) (per 10 ml/m2 increments) | 1.1 (1.0–1.2) | 0.08 | --- | --- | 1.0 (0.9–1.1) | 0.70 |
| Presence of CMR LGE (vs absence) | 7.1 (2.0–25.4) | 0.002 | 8.6 (2.4–31.6) | 0.001 | 8.2 (2.2–30.9) | 0.002 |
| Excluding patients with CAD | ||||||
| Age (5 year increments) | 1.2 (0.9–1.6) | 0.17 | --- | --- | --- | --- |
| Gender | 1.9 (0.5–6.8) | 0.33 | --- | --- | --- | --- |
| NYHA functional class | 1.8 (0.8–4.4) | 0.17 | --- | --- | 2.1 (0.8–5.5) | 0.15 |
| CMR LVEDV index (ml/m2) (per 10 ml/m2 increments) | 1.1 (1.0–1.2) | 0.14 | --- | --- | 1.0 (0.9–1.1) | 0.9 |
| Presence of CMR LGE (vs absence) | 8.9 (2.4–32.2) | 0.001 | 8.9 (2.4–32.2) | 0.001 | 9.2 (2.4–35.1) | 0.001 |
For Model 1 (parsimonious model), forward stepwise regression (p<0.10 for entry, p>0.05 for removal) was used to arrive at a parsimonious model.
For Model 2, NYHA functional class, CMR LVEDV index, and LGE fibrosis were all entered into the multivariate model.
Among patients with LGE, even small amounts of LGE were associated with a significant risk of an index adverse outcome: for the 13 patients whose % LV LGE mass measured below the median, the multivariate adjusted hazard ratio was 6.7 (95% CI 1.4–31.8, p=0.016), compared to a hazard ratio of 11.9 (95% CI 2.1–66.8, p=0.005) for those with values above the median. The rates of index adverse outcome occurrence were similar among the 3 qualitative segmental patterns of LGE: 38% of patients with a mid-wall pattern, 43% of those with a transmural pattern, and 50% of those with patchy LGE had an index outcome event. Excluding those with the transmural pattern did not significantly change the univariate and multivariate hazard ratios for LGE.
DISCUSSION
Our study found that among patients with NICM, the presence of LGE on CMR, irrespective of extent or segmental pattern, is independently associated with an adverse cardiac prognosis. After controlling for LV volume, mass or ejection fraction, and functional class, the adjusted hazard ratio for patients with CMR LGE was 8.2 (95% CI 2.2–30.9, p=0.002) for an index composite cardiac event compared to those without LGE.
The CMR LGE patterns seen in our patients are consistent with those reported in prior pathologic studies of NICM (9). Prior studies examining myocardial tissue samples obtained at the time of autopsy or cardiac transplantation have shown a pattern of increasing fibrosis from the epicardium to endocardium with both septal and LV free wall involvement (20–22). Segmental and replacement fibrosis (23) have been described in as many as 57% of patients (21) and grossly visible scars have been noted in 23% (24). The frequency and pattern of CMR LGE in our patients are consistent with these findings. The exact pathophysiology underlying the CMR abnormalities is uncertain, since NICM is the final common pathway for presumably multiple etiologies. In all likelihood, focal CMR LGE in this patient population is a non-specific measure of replacement and segmental fibrosis. These types of myocardial injury in NICM likely result from chronic sustained adrenergic activation that eventually leads to progressive myocyte dysfunction, apoptosis and fibroblast hyperplasia (8). In addition, the increased wall stress caused by progressive LV remodeling can result in focal myocyte necrosis due to microvascular ischemia from augmentation of metabolic demands as well as reduced capillary density from excessive fibrosis (8,25). Current CMR techniques are less likely to detect diffuse microscopic fibrosis.
Our finding of a strong association between poor cardiac prognosis and CMR LGE supports that of a more narrowly focused report by Assomull et al, who specifically studied the significance of the CMR LGE pattern of mid-wall fibrosis in 101 patients with symptomatic NICM (11). That study found focal mid-wall fibrosis sparing the endocardium in 35% of patients, with a hazard ratio of 3.1 (95% CI 1.1–8.5, p=0.03) for the combined outcome of all-cause mortality and hospitalization for any type of cardiovascular event, after adjustment for age, left ventricular function, and chamber volumes measured by CMR. Our results suggest that adverse cardiac prognosis is specifically associated with the presence of LGE, regardless of segmental pattern. Compared to the study by Assomull et al, which was performed at a regional HF referral center, our cohort may be more generalizable to the ICD-eligible NICM population at large: our patients were older, more ethnically diverse, received cardiac care from general cardiologists, and had uniformly severe LV dysfunction. In addition, patients were classified as nonischemic by coronary angiographic findings which is consistent with current clinical practice and an accurate definition of cardiomyopathy etiology for clinical research purposes (14). In so doing, we examined the significance of all CMR patterns in this group. Our study design was also prospective and concurrent, i.e. assessment of CMR LGE preceded clinical follow-up and outcome events were predefined at study onset.
Although we report significantly higher rates of adverse cardiac outcomes in NICM patients with CMR LGE, the absence of LGE did not assure freedom from malignant ventricular arrhythmias: 3 patients or 9% of those without LGE had an appropriate index ICD firing, compared to 4 patients (15%) of those with LGE. Significant arrhythmic risk in NICM patients without LGE is not surprising, since ventricular arrhythmias can be caused not only by fibrosis, with focal re-entry in regions of tissue heterogeneity (13,26–28), but also in the absence of fibrosis, by bundle-branch or interfasicular re-entry and by exaggerated spatial dispersion of electrophysiological properties and abnormal impulse initiation (27,28).
In addition, while the presence of LGE does predict ICD firings or cardiac death alone (p=0.03 by Kaplan-Meier analysis), HF hospitalization was a prominent determinant of the overall outcome. Although we did not study the mechanism by which increased CMR LGE leads to worse overall or specifically, HF prognosis, the dose-response relationship between the extent of LGE and outcome supports the hypothesis that the CMR findings reflect the transition from compensated to decompensated state which results from long-term stressors such as sustained adrenergic activation and/or the mechanical disadvantages caused by LV remodeling. The presence of even small amounts of macroscopic scar may indicate maladaption at the cellular level with myocyte apoptosis and necrosis, thus heralding the start of a progressively more symptomatic stage of the disease process. This is further suggested by the limited BNP data which indicate higher levels consistent with increased LV wall stress and interventricular pressures in patients with CMR LGE. Further investigation into the pathophysiology is required.
Our study has inherent limitations given its relatively small cohort size and follow-up period. Although we found a similar risk for patchy, transmural and mid-wall CMR LGE patterns, our sample size is likely too small for definitive conclusions about differential risk. Robust conclusions regarding the ability of LGE to predict each separate component of the combined endpoint are also limited. Patients underwent CMR at enrollment only, so the implications of temporal progression or regression of LGE or changes in regional pattern are unknown. Our CMR protocol used the inversion recovery technique, which is sensitive for replacement and segmental fibrosis but relatively insensitive for diffuse microscopic interstitial fibrosis. Our study included only patients with LVEF ≤35%, although the study by Assomull et al. (11) suggests that CMR LGE may be prognostically important for NICM patients with moderate LV systolic dysfunction, as well.
In conclusion, the presence of CMR LGE identifies a subset of NICM patients with an eight-fold higher risk of an index composite outcome of heart failure hospitalization, appropriate ICD firings and cardiac death, compared to patients without LGE. CMR scanning and interpretation are relatively straightforward and can be performed at many hospitals which routinely provide care for patients with heart disease. Future research will be needed to determine whether therapy guided by CMR findings can lower morbidity and mortality for patients with nonischemic cardiomyopathy and LGE such as with intensive heart failure therapies, and/or earlier consideration of cardiac transplantation or left ventricular assist device placement. In addition, extrapolation to patients with milder LV systolic dysfunction may better identify those patients at high clinical risk who are currently not recognized as such by global LV function and volumes alone.
Acknowledgments
This work was supported by the Donald W. Reynolds Cardiovascular Research Center at Johns Hopkins University and the National Heart, Lung, and Blood Institute, NIH (K23 HL04444 to KCW).
Drs. Wu and Lima receive research grant support from GE Healthcare Technologies.
We thank research coordinators, Angela Steinberg, BSN, Larissa Bell, BSN, and Barbara Butcher, CCRN; database manager, Eric Bukata, M.S. and CMR technologist, Terry Frank. Dr. Tomaselli is the Michel Mirowski Professor of Medicine. Dr. Marbán is currently Director of the Cedars-Sinai Heart Institute.
Abbreviations
- CMR
cardiovascular magnetic resonance
- NICM
nonischemic cardiomyopathy
- LVEF
left ventricular ejection fraction
- LVEDV
left ventricular end-diastolic volume
- LVESV
left ventricular end-systolic volume
- HF
heart failure
- SCD
sudden cardiac death
- ICD
implantable cardioverter defibrillator
- SI
signal intensity
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 4.Teuteberg JJ, Lewis EF, Nohria A, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail. 2006;12:47–53. doi: 10.1016/j.cardfail.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 6.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. Jama. 2004;292:2874–9. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 7.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 8.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–49. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 9.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 10.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–53. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 11.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Wu T-J, Ong JJC, Hwang C, et al. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. Journal of the American College of Cardiology. 1998;32:187–196. doi: 10.1016/s0735-1097(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 13.Hsia HH, Marchlinski FE. Characterization of the electroanatomic substrate for monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2002;25:1114–27. doi: 10.1046/j.1460-9592.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 14.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–8. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 15.Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–9. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–14. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II) J Am Coll Cardiol. 2004;43:1459–65. doi: 10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 19.Bart BA, Shaw LK, McCants CB, Jr, et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol. 1997;30:1002–8. doi: 10.1016/s0735-1097(97)00235-0. [DOI] [PubMed] [Google Scholar]
- 20.Unverferth DV, Baker PB, Swift SE, et al. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. Am J Cardiol. 1986;57:816–20. doi: 10.1016/0002-9149(86)90620-x. [DOI] [PubMed] [Google Scholar]
- 21.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340–55. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 22.de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2001;14:299–306. doi: 10.1007/s001470100339. [DOI] [PubMed] [Google Scholar]
- 23.Beltrami CA, Finato N, Rocco M, et al. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27:291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- 24.Waller TA, Hiser WL, Capehart JE, Roberts WC. Comparison of clinical and morphologic cardiac findings in patients having cardiac transplantation for ischemic cardiomyopathy, idiopathic dilated cardiomyopathy, and dilated hypertrophic cardiomyopathy. Am J Cardiol. 1998;81:884–94. doi: 10.1016/s0002-9149(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 25.Baig MK, Mahon N, McKenna WJ, et al. The pathophysiology of advanced heart failure. American Heart Journal. 1998;135:S216–S230. doi: 10.1016/s0002-8703(98)70252-2. [DOI] [PubMed] [Google Scholar]
- 26.Nazarian S, Bluemke DA, Lardo AC, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–5. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delacretaz E, Stevenson WG, Ellison KE, Maisel WH, Friedman PL. Mapping and radiofrequency catheter ablation of the three types of sustained monomorphic ventricular tachycardia in nonischemic heart disease. J Cardiovasc Electrophysiol. 2000;11:11–7. doi: 10.1111/j.1540-8167.2000.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 28.Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation. 1998;98:2404–14. doi: 10.1161/01.cir.98.22.2404. [DOI] [PubMed] [Google Scholar]