Abstract
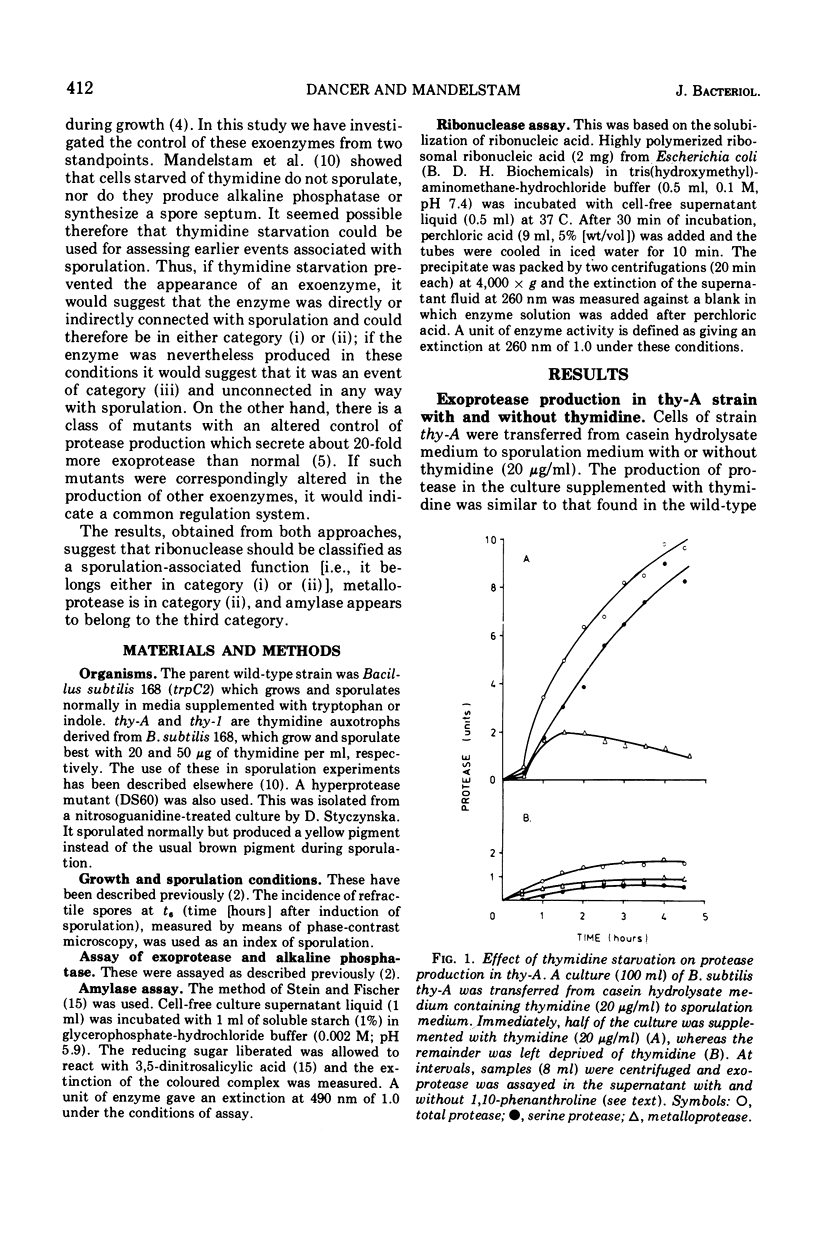
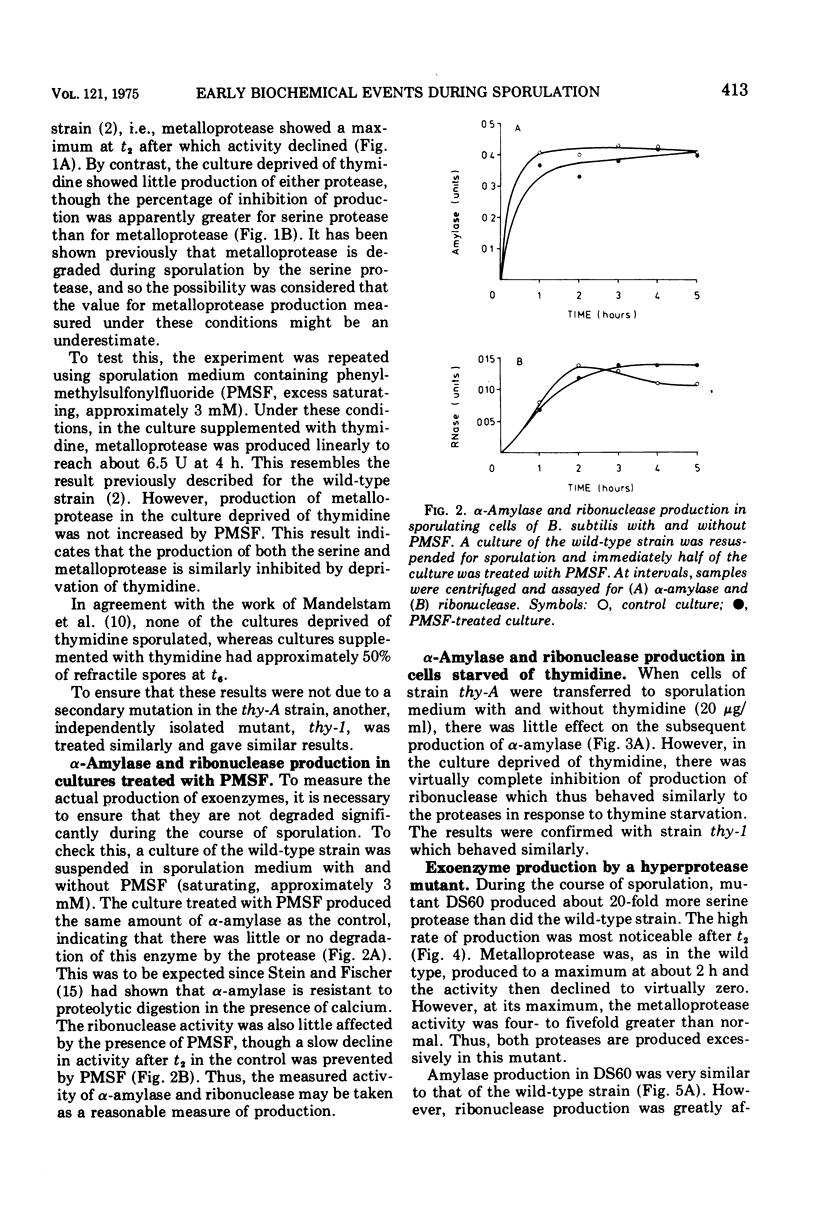
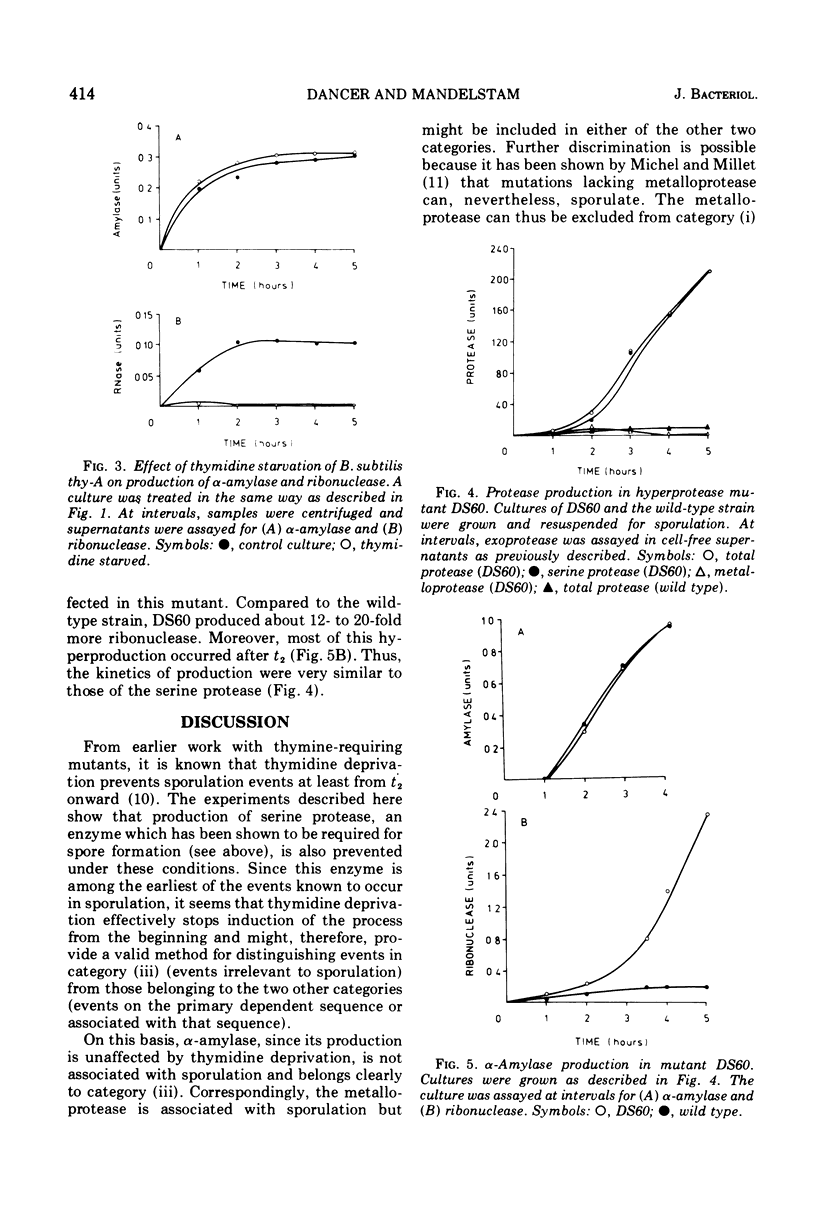
Two criteria are suggested for assessing the relevance of biochemical events occurring early in sporulation. The first is thymidine starvation, a condition known to inhibit sporulation. This also inhibits the production of metalloprotease, serine protease, and ribonuclease; alpha-amylase production, however, is unaffected. The second is the effect of a regulator mutation which increases the production of the proteases. In the mutant, ribonuclease is produced in correspondingly large quantities whereas alpha-amylase production is unaffected. We conclude that, whereas the serine protease is part of the main sequence of events leading to formation of the spore, the metalloprotease is a side effect, i.e., connected with the main sequence but not part of it. Ribonuclease could, on present evidence, be either in the main sequence or a side effect associated with it. Amylase, however, seems to be separately regulated and neither directly nor indirectly connected with the sporulation sequence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coote J. G., Mandelstam J. Use of constructed double mutants for determining the temporal order of expression of sporulation genes in Bacillus subtilis. J Bacteriol. 1973 Jun;114(3):1254–1263. doi: 10.1128/jb.114.3.1254-1263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer B. N., Mandelstam J. Production and possible function of serine protease during sporulation of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):406–410. doi: 10.1128/jb.121.2.406-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. Effects of mutational loss of specific intracellular proteases on the sporulation of Bacillus subtilis. J Bacteriol. 1973 May;114(2):612–617. doi: 10.1128/jb.114.2.612-617.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineken F. G., O'Connor R. J. Continuous culture studies on the biosynthesis of alkaline protease, neutral protease and -amylase by Bacillus subtilis NRRL-B3411. J Gen Microbiol. 1972 Nov;73(1):35–44. doi: 10.1099/00221287-73-1-35. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Hoch J. A., Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):1026–1028. doi: 10.1128/jb.112.2.1026-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Chien J. R., Lehman I. R. Exonucleolytic degradation of high molecular weight deoxyribonucleic acid and ribonucleic acid to nucleoside 3'-phosphates by a nuclease from Bacillus subtilis. J Biol Chem. 1967 Jun 10;242(11):2700–2708. [PubMed] [Google Scholar]
- Lanyi J. K., Lederberg J. Fluorescent method for the detection of excreted ribonuclease around bacterial colonies. J Bacteriol. 1966 Nov;92(5):1469–1472. doi: 10.1128/jb.92.5.1469-1472.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J., Sterlini J. M., Kay D. Sporulation in Bacillus subtilis. Effect of medium on the form of chromosome replication and on initiation to sporulation in Bacillus subtilis. Biochem J. 1971 Nov;125(2):635–641. doi: 10.1042/bj1250635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J. F., Millet J. Physiological studies on early-blocked sporulation mutants of Bacillus subtilis. J Appl Bacteriol. 1970 Mar;33(1):220–227. doi: 10.1111/j.1365-2672.1970.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN E. A., FISCHER E. H. The resistance of alpha-amylases towards proteolytic attack. J Biol Chem. 1958 Jun;232(2):867–879. [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


