Abstract
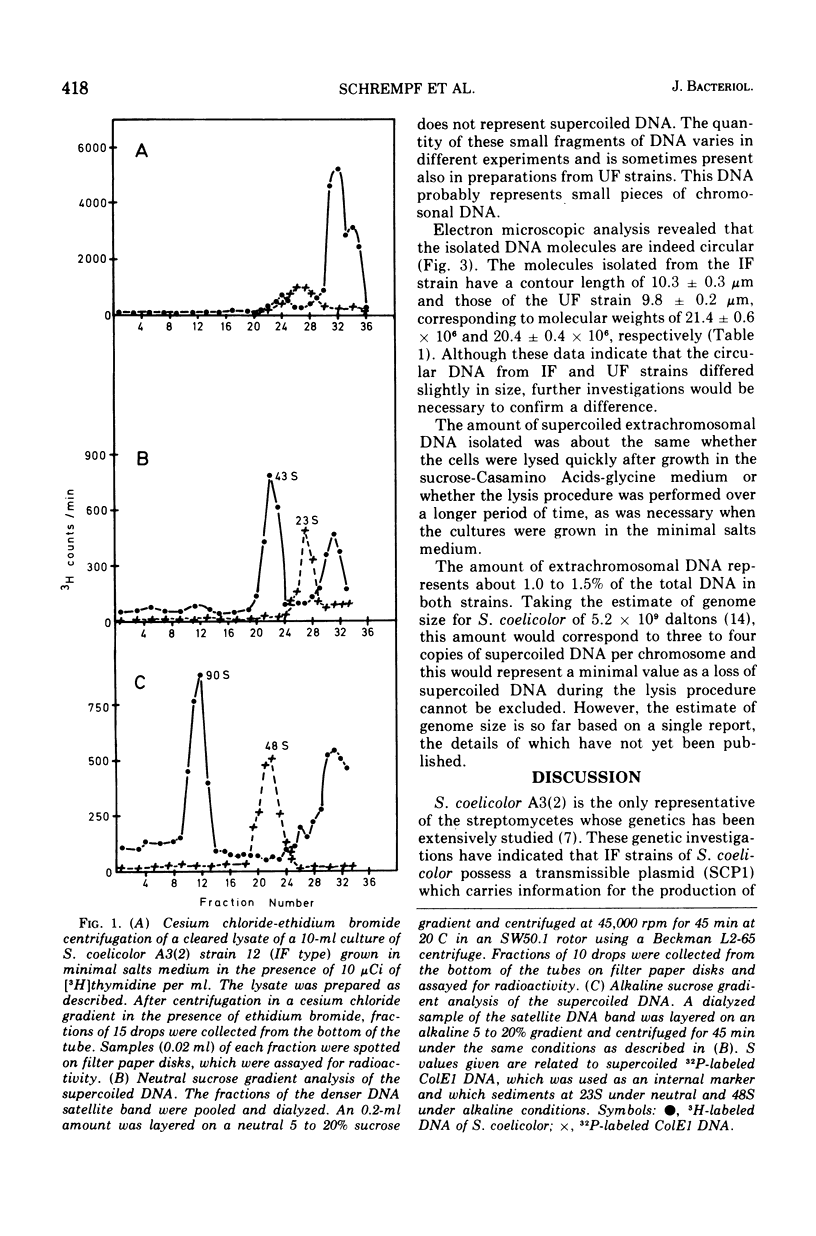
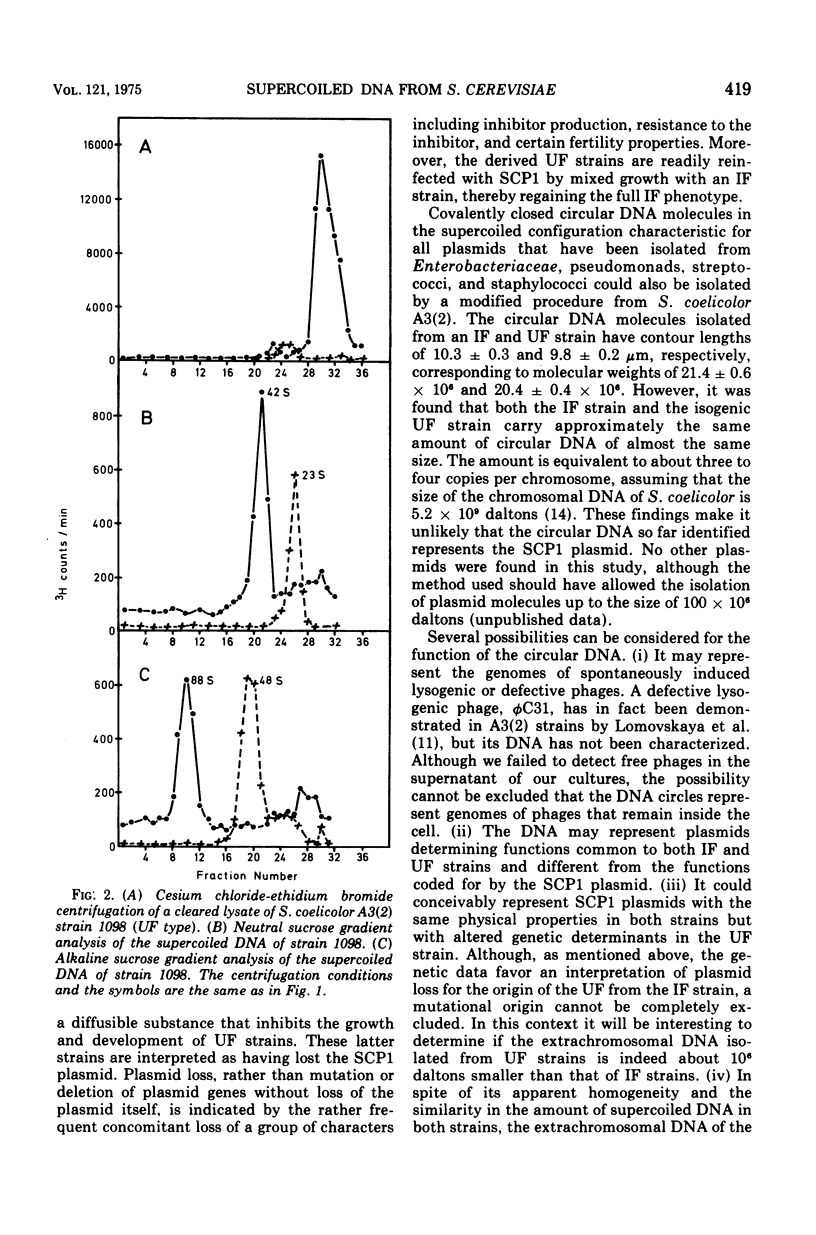
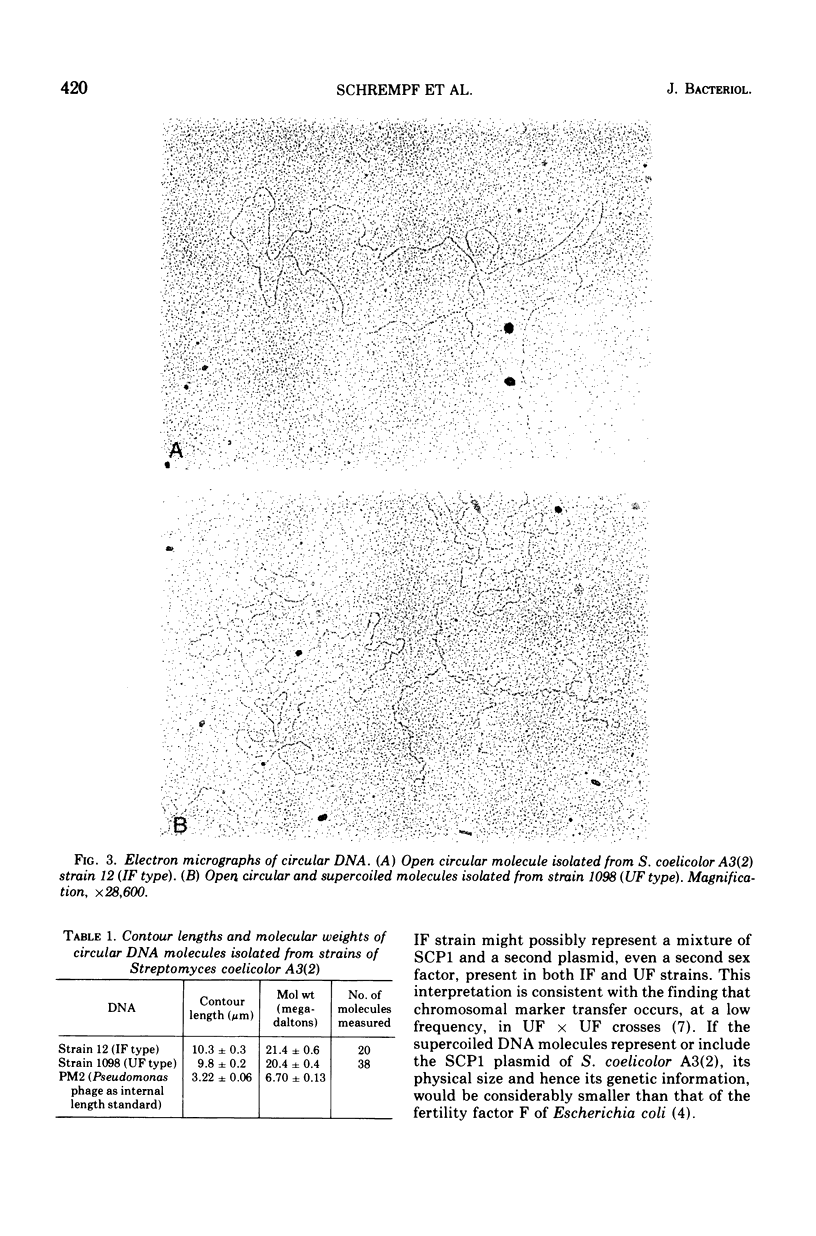
Covalently closed circular deoxyribonucleic acid (DNA) was isolated from two strains of Streptomyces coelicolor A3(2), representing two of the known fertility types. In each of the two strains circular DNA of about 20 times 10-6 daltons could be detected, amounting to about 1.5% of the total cellular DNA. The possible function of this DNA is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bujard H. Electron microscopy of single-stranded DNA. J Mol Biol. 1970 Apr 14;49(1):125–137. doi: 10.1016/0022-2836(70)90381-5. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Vinograd J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature. 1967 Nov 18;216(5116):652–657. doi: 10.1038/216652a0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. II. Some physical properties of F'Lac and F DNA. J Mol Biol. 1968 Feb 28;32(1):25–35. doi: 10.1016/0022-2836(68)90142-3. [DOI] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. II. INDUCTION OF TYROSINASE DEFICIENCY BY ACRIDINE DYES. J Bacteriol. 1964 Jun;87:1287–1294. doi: 10.1128/jb.87.6.1287-1294.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Chater K. F., Dowding J. E., Vivian A. Advances in Streptomyces coelicolor genetics. Bacteriol Rev. 1973 Sep;37(3):371–405. doi: 10.1128/br.37.3.371-405.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. A plasmid of Streptomyces coelicolor carrying a chromosomal locus and its inter-specific transfer. J Gen Microbiol. 1973 Dec;79(2):331–342. doi: 10.1099/00221287-79-2-331. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi M., Ita T., Umezawa H. Possible control of formation of aerial mycelium and antibiotic production in Streptomyces by episomic factors. J Antibiot (Tokyo) 1970 Jan;23(1):45–47. doi: 10.7164/antibiotics.23.45. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Suzuki K., Umezawa H. Formation and reversion of Streptomycete protoplasts: cultural condition and morphological study. J Gen Microbiol. 1974 Feb;80(2):389–400. doi: 10.1099/00221287-80-2-389. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Identification of individual sex-factor DNA strands and their replication during conjugation in thermosensitive DNA mutants of Escherichia coli. J Mol Biol. 1971 Sep 28;60(3):413–424. doi: 10.1016/0022-2836(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Vivian A., Hopwood D. A. Genetic control of fertility in Streptomyces coelicolor A3(2): the IF fertility type. J Gen Microbiol. 1970 Nov;64(1):101–117. doi: 10.1099/00221287-64-1-101. [DOI] [PubMed] [Google Scholar]



