Abstract
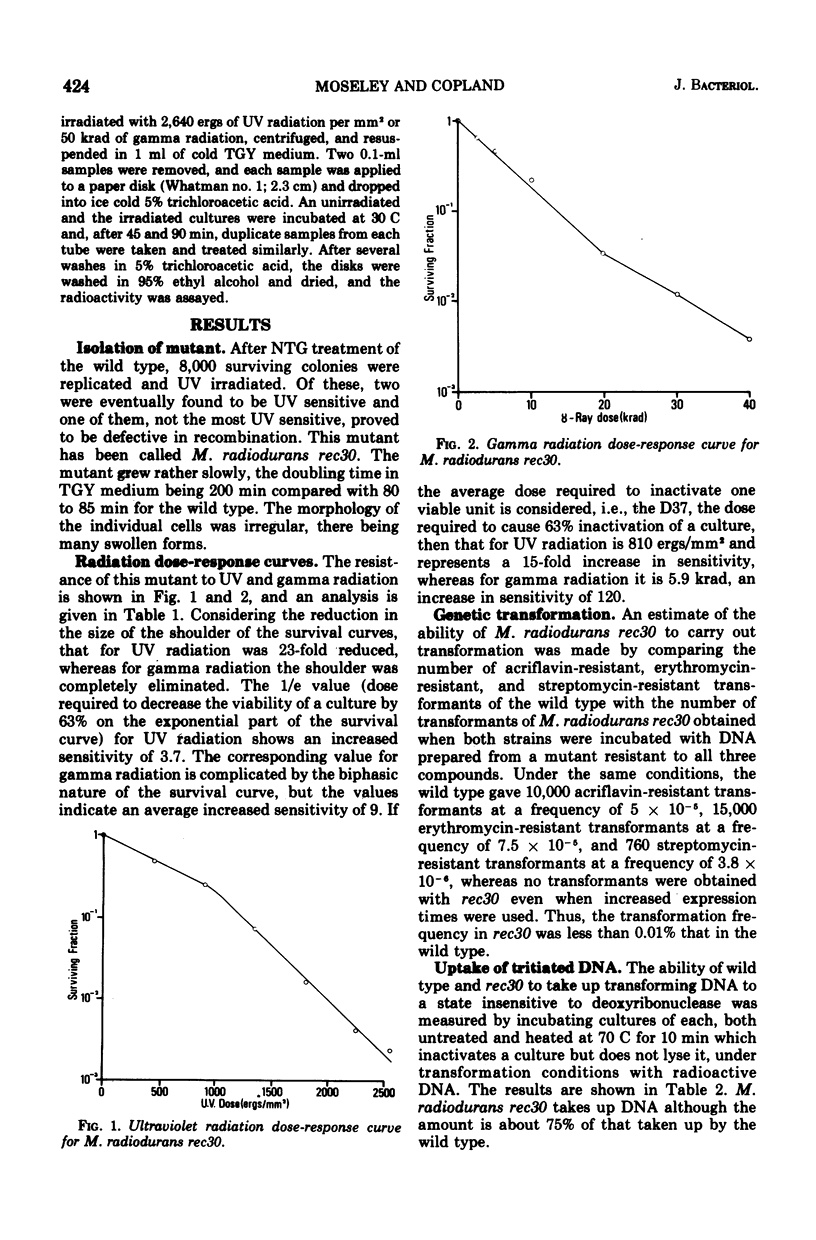
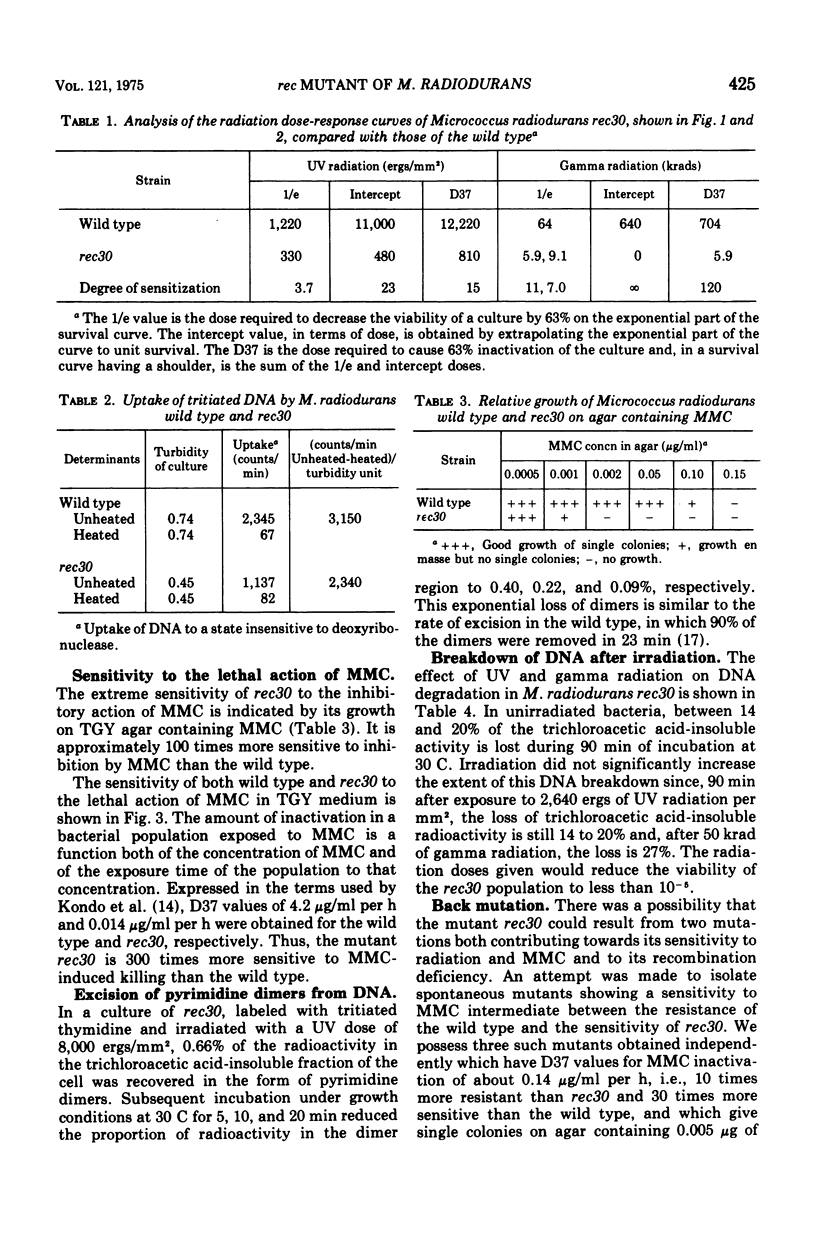
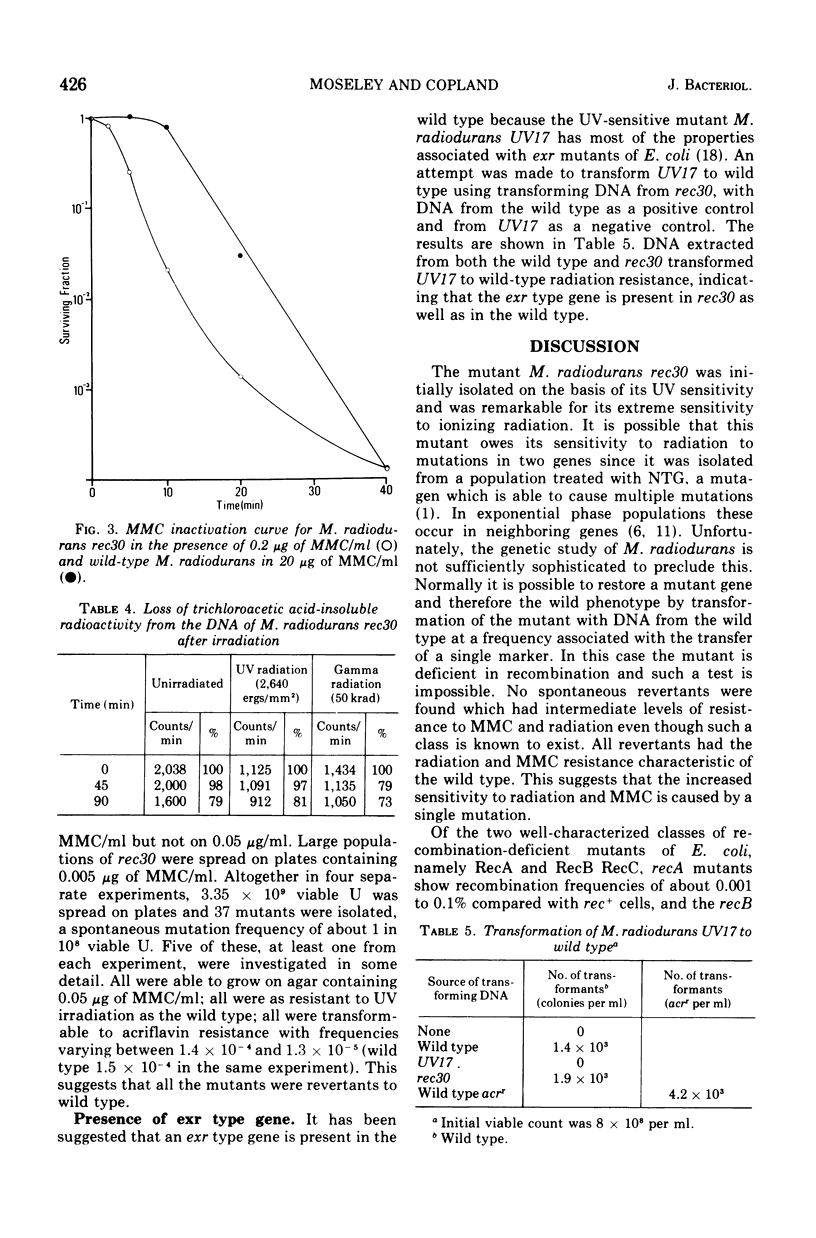
A mutant of micrococcus radiodurans which is deficient in recombination has been isolated after treatment of the wild type with N-methyl-N'-nitro-N-nitrosoguanidine. We have called this mutant Micrococcus radiodurans rec30. The efficiency of recombination in this mutant, as measured by transformation, is less than 0.01% that of the wild type. It is 15 times more sensitive to the lethal action of ultraviolet radiation, 120 times more sensitive to ionizing radiation, and 300 times more sensitive to mitomycin C (MMC) than the wild type. It is probably inactivated by a single MMC-induced deoxyribonucleic acid cross-link per genome. The excision of ultraviolet-induced pyrimidine dimers is normal. There is no radiation-induced degradation of deoxyribonucleic acid. All spontaneous revertants selected for resistance to low levels of MMC had wild-type resistance to radiation and MMC, and the same efficiency of recombination as the wild type, suggesting that the recombination deficiency of the strain is due to a single mutation. Deoxyribonucleic acid from this mutant can transform M. radiodurans UV17 presumed deficient in an exr type gene to wild type.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J Bacteriol. 1971 Sep;107(3):846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger A. A. The DNA content of single cells of Micrococcus radiodurans. Can J Microbiol. 1970 Nov;16(11):1136–1137. doi: 10.1139/m70-192. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E., Mattingly A., Copland H. J. Sensitization to radiation by loss of recombination ability in a temperature-sensitive DNA mutant of Micrococcus radiodurans held at its restrictive temperature. J Gen Microbiol. 1972 Sep;72(2):329–338. doi: 10.1099/00221287-72-2-329. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Mattingly A. Repair of irradiation transforming deoxyribonucleic acid in wild type and a radiation-sensitive mutant of Micrococcus radiodurans. J Bacteriol. 1971 Mar;105(3):976–983. doi: 10.1128/jb.105.3.976-983.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E. Repair of ultraviolet radiation damage in sensitive mutants of Micrococcus radiodurans. J Bacteriol. 1969 Feb;97(2):647–652. doi: 10.1128/jb.97.2.647-652.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E. The isolation and some properties of radiation-sensitive mutants of Micrococcus radiodurans. J Gen Microbiol. 1967 Nov;49(2):293–300. doi: 10.1099/00221287-49-2-293. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


