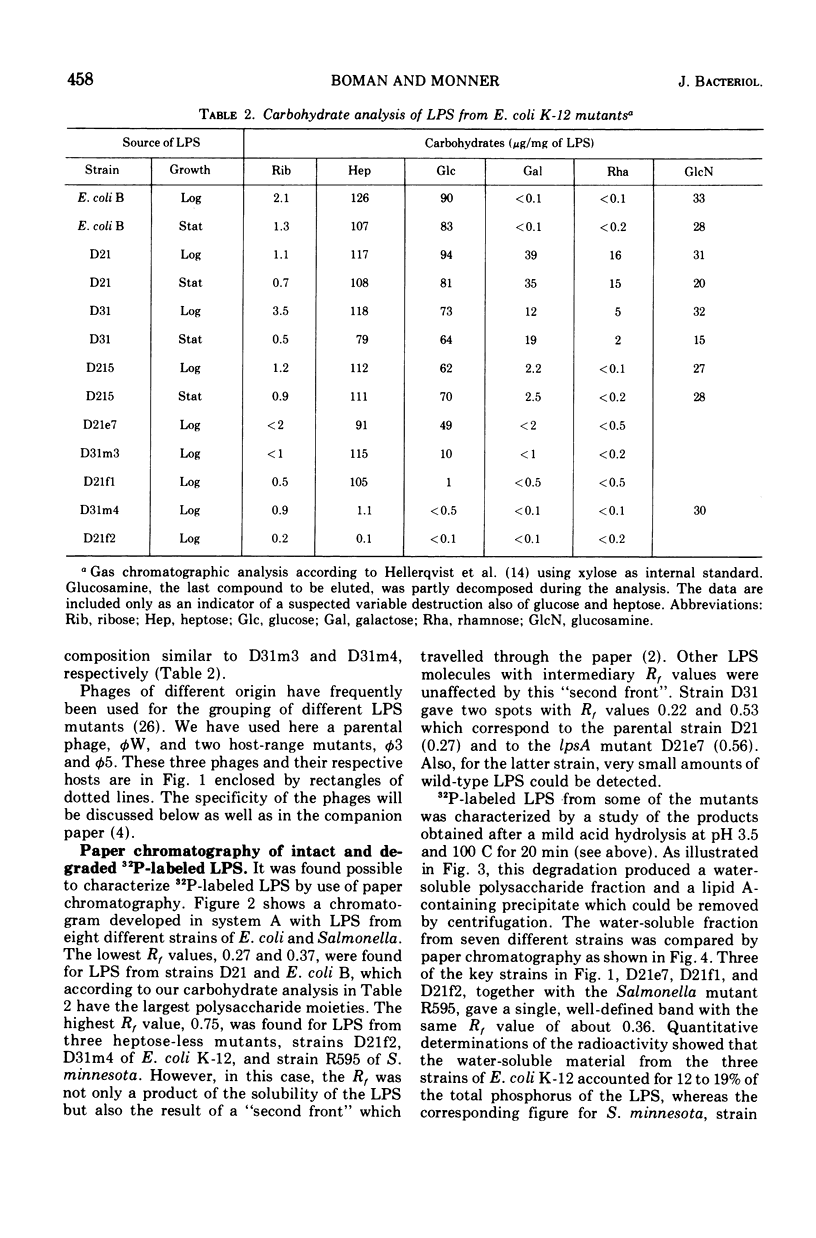
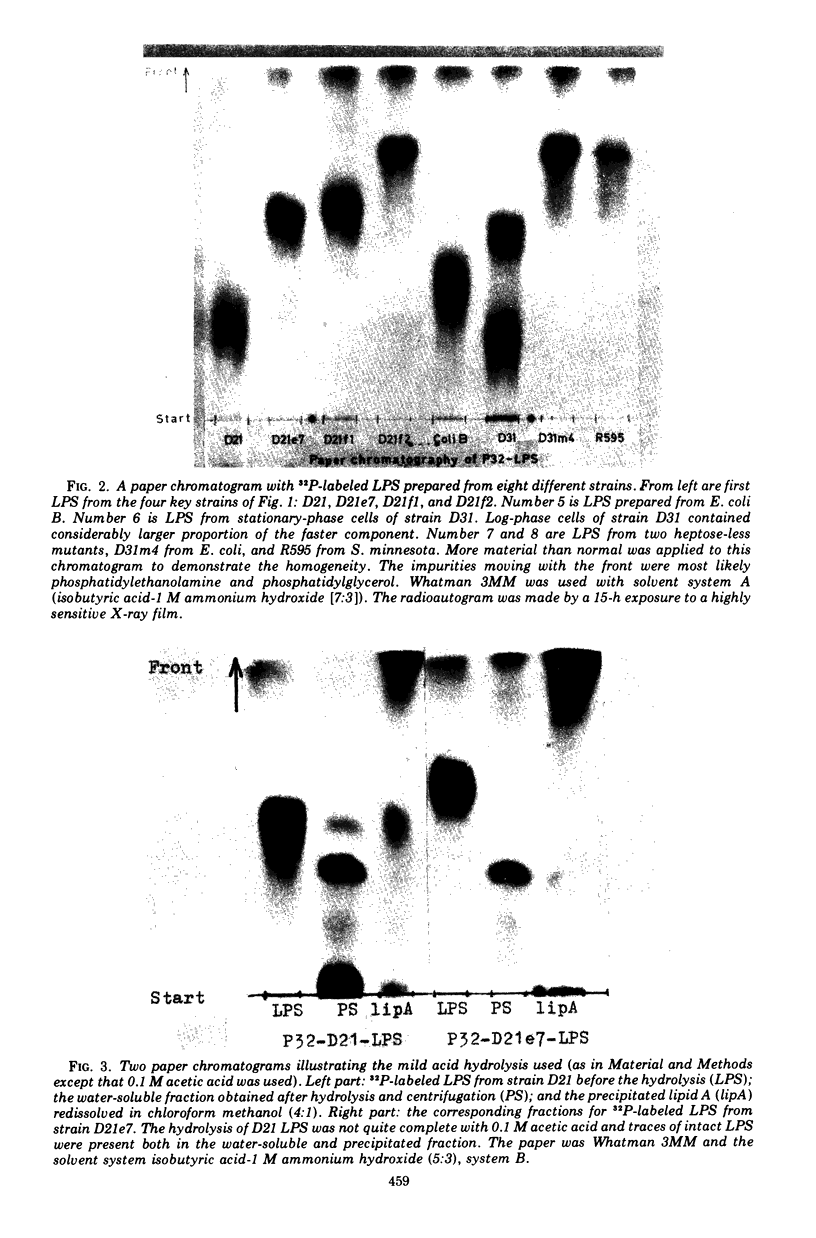
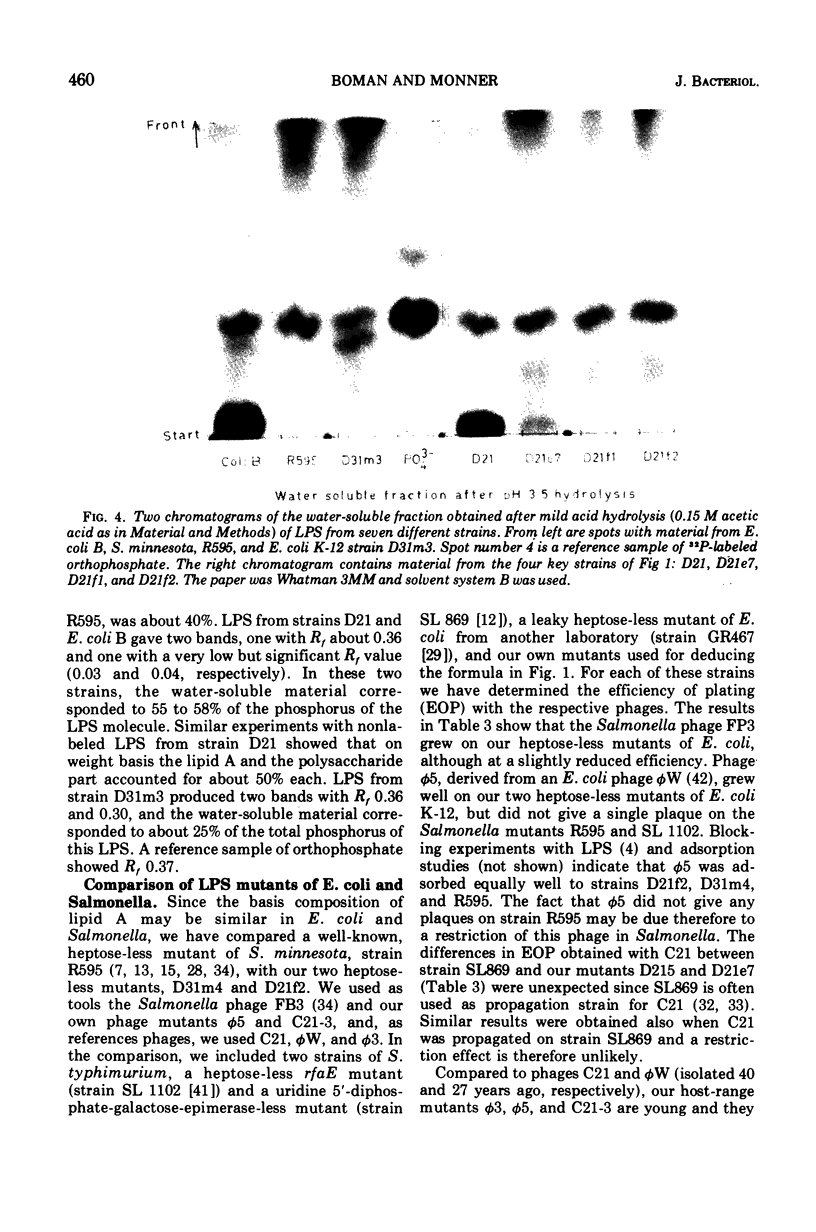
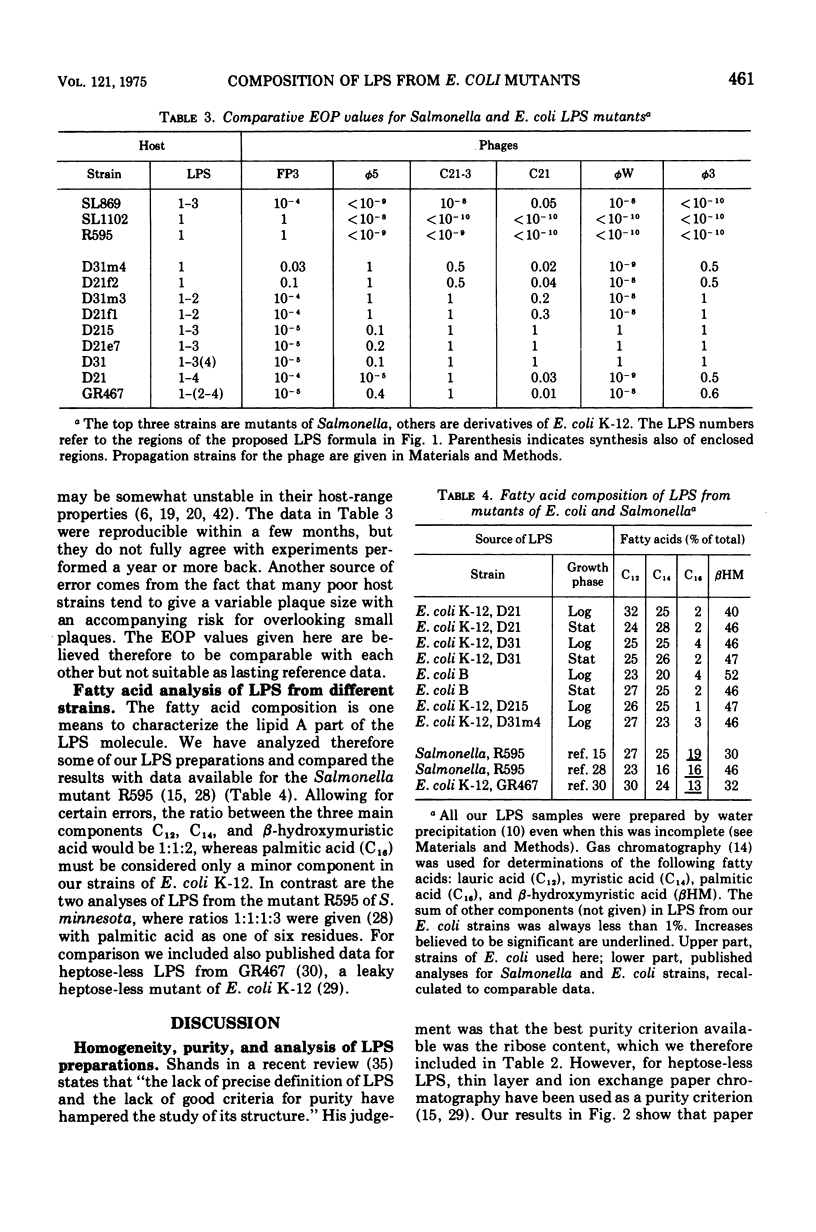
Abstract
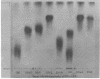
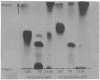
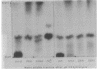
Chemical analyses of the carbohydrate composition of lipopolysaccharides (LPS) from a number of LPS mutants were used to propose a schematic composition for the LPS from Escherichia coli K-12. The formula contains four regions: the first consists of lipid A, ketodeoxyoctonoic acid, and a phosphorous component; the second contains only heptose; the third only glucose; and the fourth additional glucose, galactose, and rhamnose. LPS from E. coli B may have a similar composition but lacks the galactose and rhamnose units. A set of LPS-specific bacteriophages were used for comparing three mutants of Salmonella with a number of LPS mutants of E. coli K-12. The results confirm that there are basic similarities in the first and second regions of the LPS structure; they also support the four region divisions of the LPS formula. Paper chromatography was used for characterization of 32-P-labeled LPS from different strains of E. coli and Salmonella. The Rf values for LPS varied from 0.27 to 0.75 depending on the amounts of carbohydrates in the molecule. LPS from all strains studied was homogenous except for strain D31 which produced two types of LPS. Mild acid hydrolysis of labeled LPS liberated lipid A and two other components with phosphate, one of which was assigned to the first region. It is suggested that paper chromatography can be used in biosynthetic studies concerning regions 2 to 4.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMAN H. G. Double fronting in paper chromatography of proteins. Nature. 1952 Oct 25;170(4330):703–704. doi: 10.1038/170703a0. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Monner D. A. Blocking of bacteriophages phi W and phi 5 with lipopolysaccharides from Escherichia coli K-12 mutants. J Bacteriol. 1975 Feb;121(2):465–470. doi: 10.1128/jb.121.2.465-470.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. R., Nordström K., Englund P. Resistance of Escherichia coli to penicillins. IX. Genetics and physiology of class II ampicillin-resistant mutants that are galactose negative or sensitive to bacteriophage C21, or both. J Bacteriol. 1971 Dec;108(3):1210–1223. doi: 10.1128/jb.108.3.1210-1223.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller N. A., Wu M., Wilkinson R. G., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VII. Characterization of heterogeneous "core" oligosaccharide structures. J Biol Chem. 1973 Nov 25;248(22):7938–7950. [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Stocker B. A. Transduction by bacteriophage P22 in nonsmooth mutants of Salmonella typhimurium. J Bacteriol. 1967 May;93(5):1588–1597. doi: 10.1128/jb.93.5.1588-1597.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 6. Investigations on the structure of the lipid A component. Eur J Biochem. 1969 Jan;7(3):370–379. doi: 10.1111/j.1432-1033.1969.tb19618.x. [DOI] [PubMed] [Google Scholar]
- Kasai N., Nowotny A. Endotoxic glycolipid from a heptoseless mutant of Salmonella minnesota. J Bacteriol. 1967 Dec;94(6):1824–1836. doi: 10.1128/jb.94.6.1824-1836.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann V., Lüderitz O., Westphal O. The linkage of pyrophosphorylethanolamine to heptose in the core of Salmonella minnesota lipopolysaccharides. Eur J Biochem. 1971 Aug 16;21(3):339–347. doi: 10.1111/j.1432-1033.1971.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monner D. A., Boman H. G. Female strains of Escherichia coli K12 as selective hosts for the isolation of female specific mutants of phage omega II. Biochem Biophys Res Commun. 1970;39(6):1017–1020. doi: 10.1016/0006-291x(70)90659-5. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Boman H. G. Host-dependent properties of coliphage phiW and its female-specific host-range mutants phi3 and phi4. J Gen Virol. 1974 Jul;24(1):67–75. doi: 10.1099/0022-1317-24-1-67. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. J., Stewart J. C. Structural studies on the lipopolysaccharide of a rough strain of Escherichia coli. Eur J Biochem. 1972 Sep 18;29(2):308–318. doi: 10.1111/j.1432-1033.1972.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Rapin A. M., Kalckar H. M., Alberico L. The metabolic basis for masking of receptor-sites on E. coli K-12 for C21, a lipopolysaccharide core-specific phage. Arch Biochem Biophys. 1968 Oct;128(1):95–105. doi: 10.1016/0003-9861(68)90011-8. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H. Isolation and characterization of 2-keto-3-deoxyoctonate-lipid A from a heptose-deficient mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):531–541. doi: 10.1128/jb.111.2.531-541.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H., Sweeley C. C. The identification of trans-2-tetradecenoic acid in hydrolysates of lipid A from Escherichia coli. Biochim Biophys Acta. 1972 Jul 7;270(3):289–295. doi: 10.1016/0005-2760(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Fromme I., Mayer H. Immunochemical studies on core lipopolysaccharides of Enterobacteriaceae of different genera. Eur J Biochem. 1970 Jun;14(2):357–366. doi: 10.1111/j.1432-1033.1970.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G. Genetical studies on the lipopolysaccharide structure of Escherichia coli K12. J Gen Microbiol. 1973 Jul;77(1):151–160. doi: 10.1099/00221287-77-1-151. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Lüderitz O. Untersuchungen zur Typisierung von Salmonella-R-Formen. II. Typisierung von S. minnesota-Mutanten durch Phagen. Zentralbl Bakteriol Orig. 1969 Jul;210(3):381–387. [PubMed] [Google Scholar]
- Sugimoto K., Okazaki R. A new rhamnosyl compound in lipopolysaccharide preparations of Escherichia coli. Isolation and enzymatic synthesis. J Biochem. 1967 Sep;62(3):373–383. [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]