Abstract
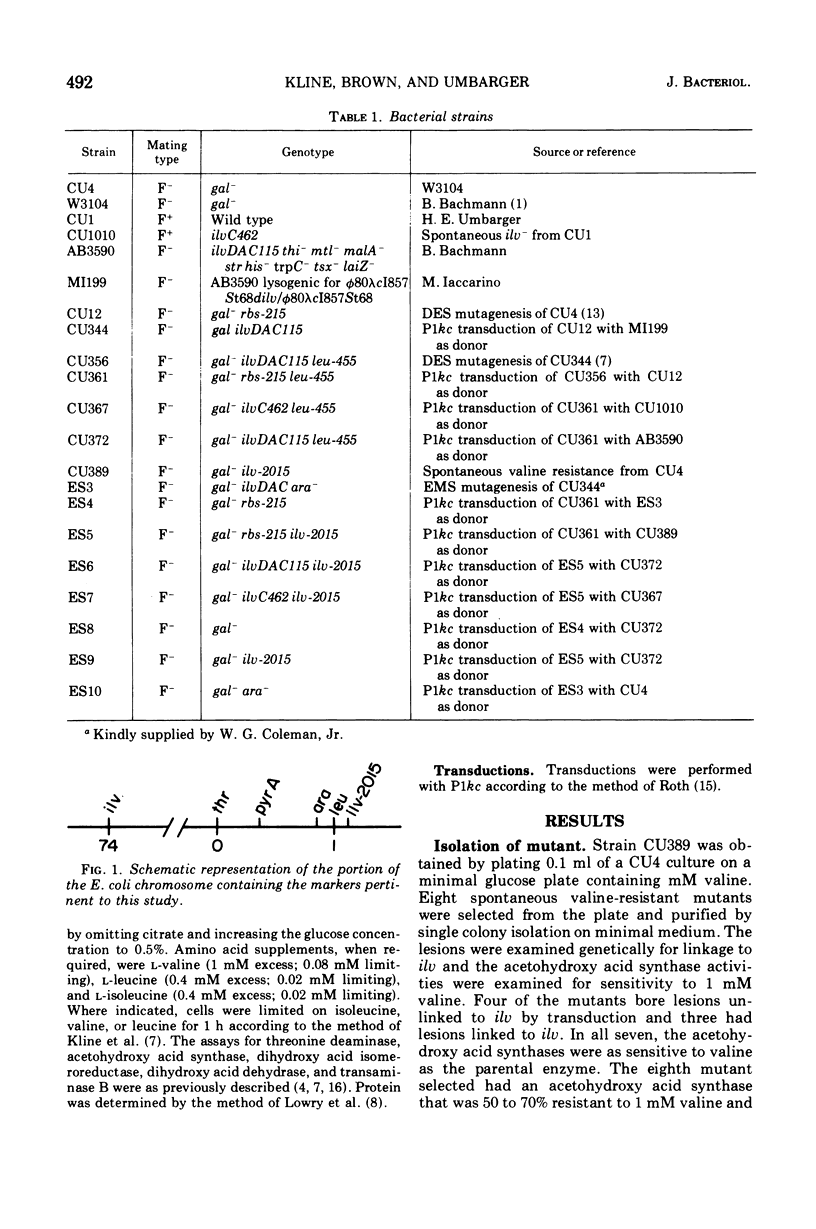
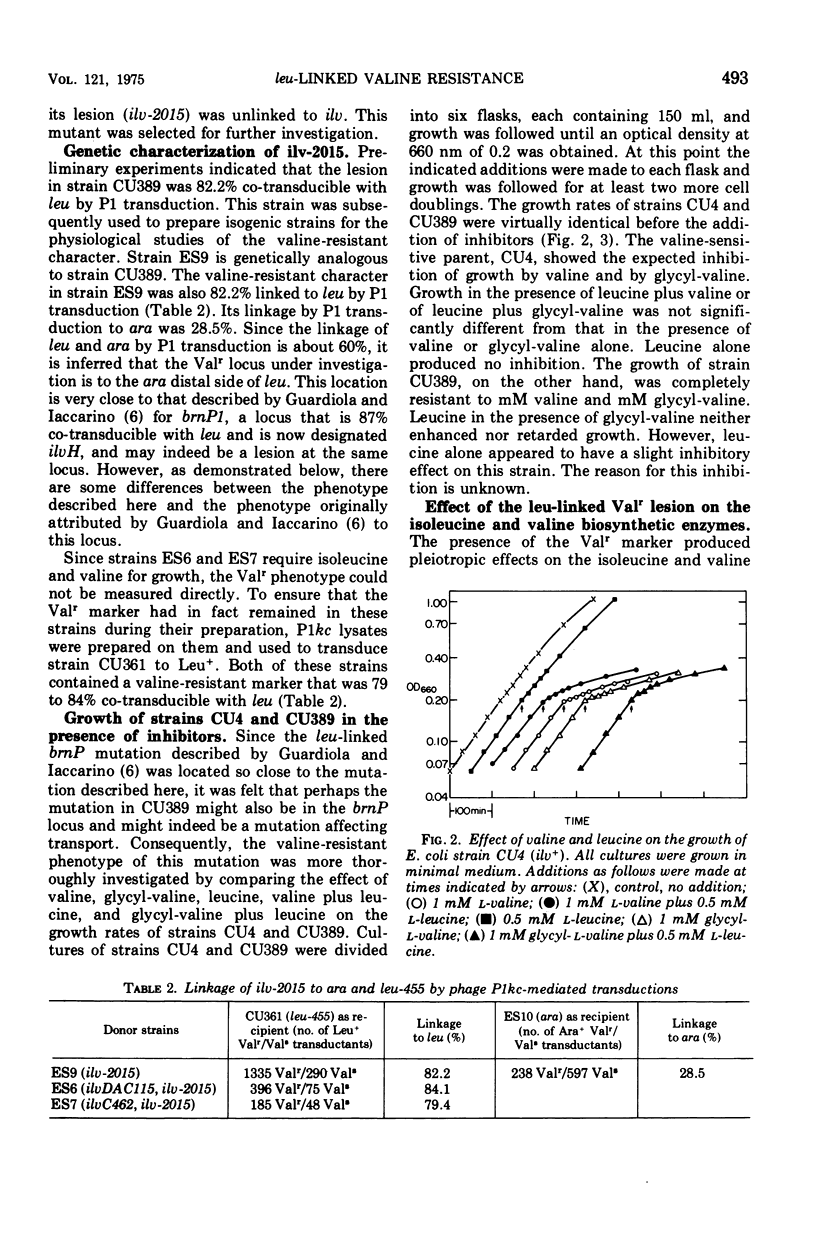
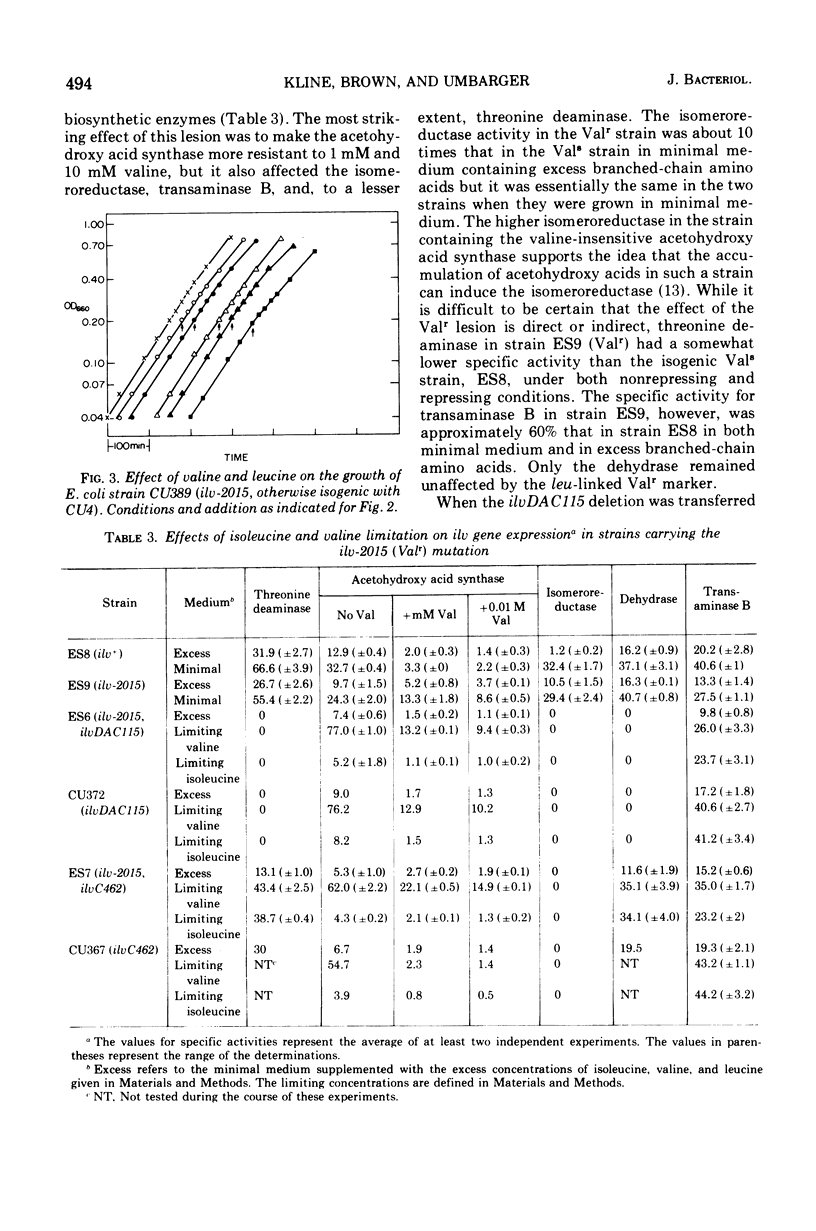
A spontaneous leu-linked mutation (ilvH2015) in Escherichia coli K-12 made the strain resistant to 1 mM valine and l mM glycylvaline (Val-r) and caused the isoleucine and valine biosynthetic enzyme, acetohydroxy acid synthase, to be less sensitive to feedback inhibition by valine than the wild-type enzyme. Transfer of the ilvDAC deletion into a strain carrying ilvH2015 abolished the effect of the marker on the acetohydroxy acid synthase and rendered it as sensitive to valine as the enzyme in the isogenic control strain without the Val-r marker under both repressing and limiting conditions. In contrast, auxotrophy caused by transfer of an ilvC lesion into the Val-r strain did not interfere with the effect of ilvH2015 on valine sensitivity of acetohydroxy acid synthase. In addition, the presence of the Val-r marker produced minor but significant pleiotropic effects on several other isoleucine and valine biosynthetic enzymes but did not cause derepression of the ilv gene cluster. These studies suggest some type of interaction between a product produced by a gene close to leu and the isoleucine and valine biosynthetic enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt J. M., Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XX. Multiple forms of acetohydroxy acid synthetase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):444–450. doi: 10.1016/s0006-291x(72)80071-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- Guardiola J., Iaccarino M. Escherichia coli K-12 mutants altered in the transport of branched-chain amino acids. J Bacteriol. 1971 Dec;108(3):1034–1044. doi: 10.1128/jb.108.3.1034-1044.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Coleman W. G., Jr, Umbarger H. E. Regulation of isoleucine-valine biosynthesis in an ilvDAC deletion strain of Escherichia coli K-12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1144–1151. doi: 10.1016/0006-291x(74)90816-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANTEN A., ROWLEY D. Genetic analysis of valine inhibition in the K12 strain of Bacterium coli. J Gen Microbiol. 1953 Oct;9(2):226–233. doi: 10.1099/00221287-9-2-226. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Two forms of biosynthetic acetohydroxy acid synthetase in Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Jul 25;48(2):437–443. doi: 10.1016/s0006-291x(72)80070-6. [DOI] [PubMed] [Google Scholar]
- PITTARD J., LOUTIT J. S., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. I. DELAY IN INITIATION OF CHROMOSOME TRANSFER. J Bacteriol. 1963 Jun;85:1394–1401. doi: 10.1128/jb.85.6.1394-1401.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXI. Mutations affecting derepression and valine resistance. J Bacteriol. 1973 Apr;114(1):183–194. doi: 10.1128/jb.114.1.183-194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


