Abstract
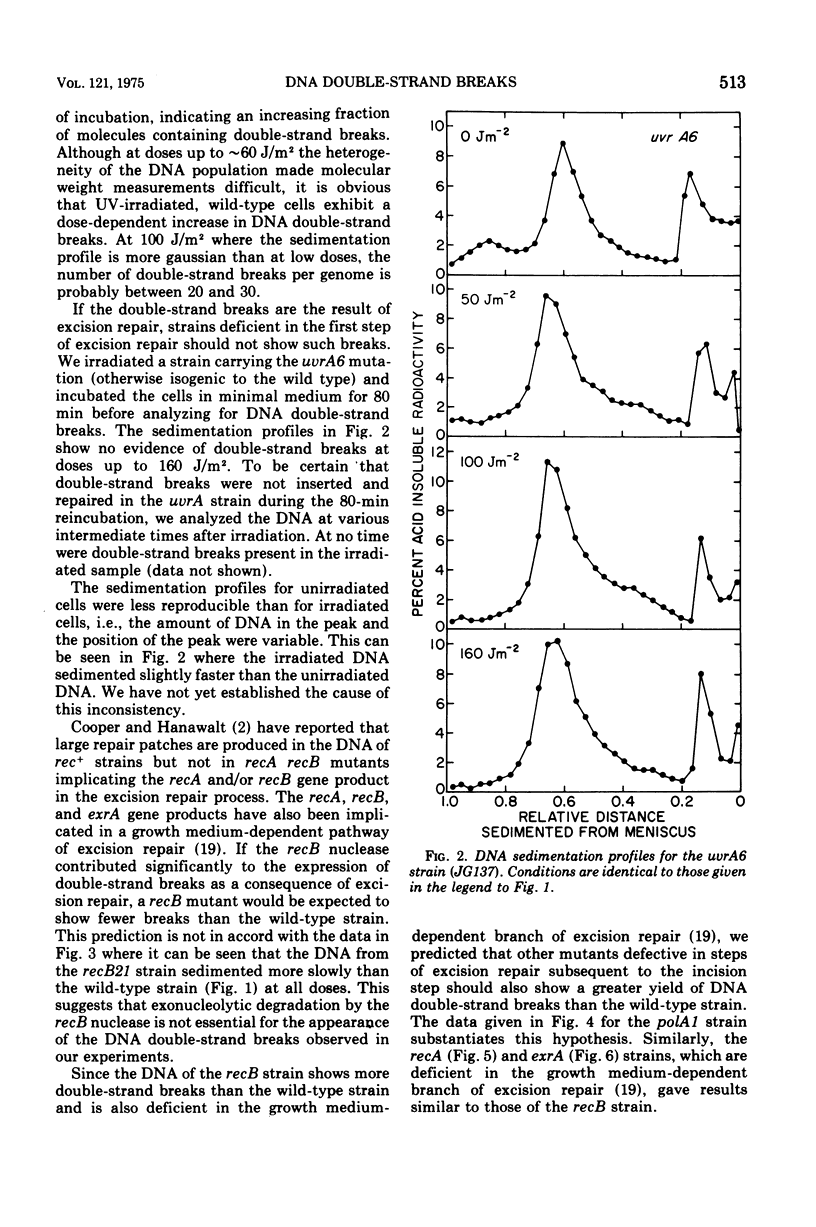
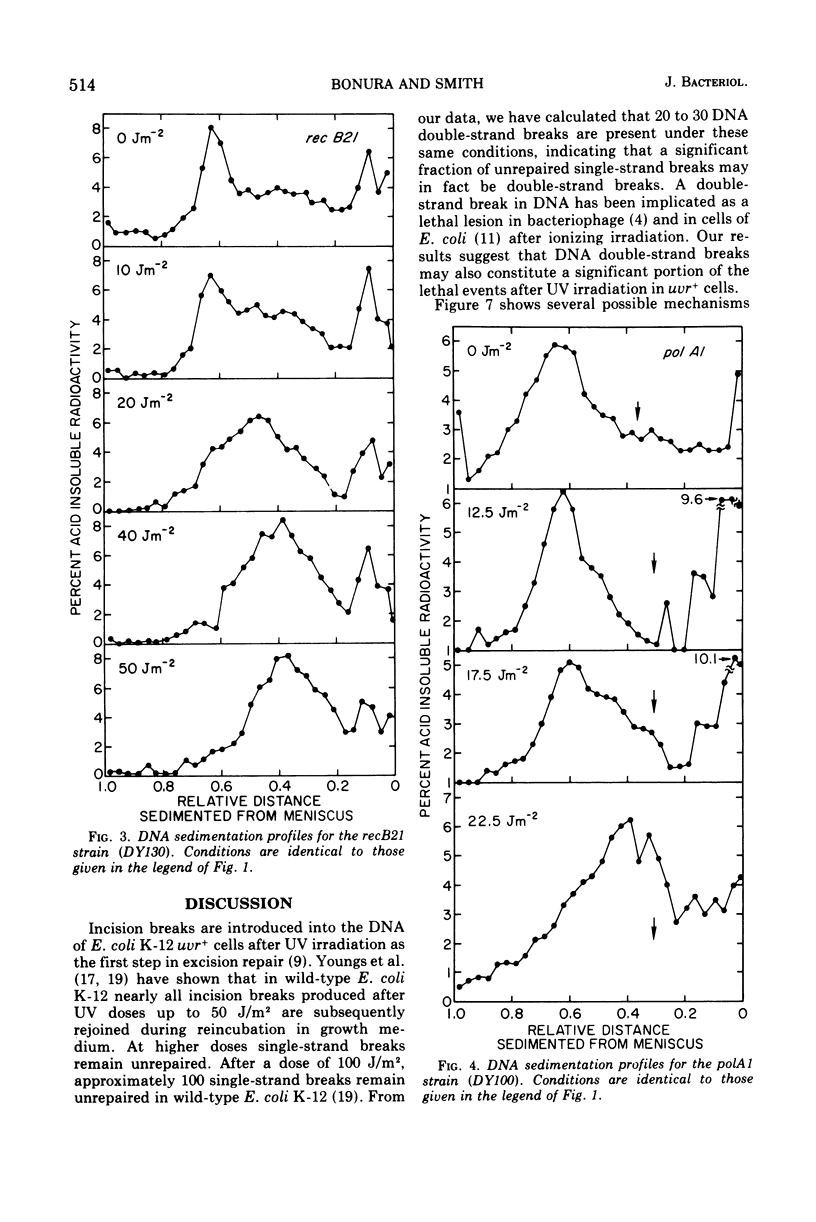
We have observed the enzymatic production of deoxyribonucleic acid (DNA) doublestrand breaks in Escherichia coli K12 after ultraviolet irradiation. Doublestrand breaks appeared in wild-type, polA1, recB21, recA, and exrA strains after incubation in minimal medium. THE UVRA6 strain showed no evidence of double-strand breakage under the same conditions. Our data suggest that uvr+ cells, which are proficient in the incision step of excision repair, accumulate double-strand breaks in their DNA as a result of the excision repair process, i.e., arising from closely matched incisions, excision gaps, or incisions and gaps on opposite strands of the DNA twin helix. Furthermore, strains deficient in excision repair subsequent to the incision step (i.e., polA, rec, exrA) showed more double-strand breaks than the wild type strain. The results raise the possibility that a significant fraction of the lethal events in ultraviolet-irradiated, repair-proficient (uvr+) cell may be enzymatically-induced DNA double-strand breaks.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Role of DNA polymerase I and the rec system in excision-repair in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1156–1160. doi: 10.1073/pnas.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Mechanism of inactivation of coliphage T7 by x-rays. Proc Natl Acad Sci U S A. 1965 Jul;54(1):128–134. doi: 10.1073/pnas.54.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Dark recovery processes in Escherichia coli irradiated with ultraviolet light. I. Effect of rec mutations on liquid holding recovery. J Bacteriol. 1968 Aug;96(2):365–373. doi: 10.1128/jb.96.2.365-373.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman B. W., van Sluis C. A., Heijneker H. L., Rörsch A. A mutant of Escherichia coli K12 deficient in the 5'-3' exonucleolytic activity of DNA polymerase I. I. General characterization. Mol Gen Genet. 1973 Jul 31;124(1):69–82. doi: 10.1007/BF00267166. [DOI] [PubMed] [Google Scholar]
- Harm W. Effects of dose fractionation on ultraviolet survival of Escherichia coli. Photochem Photobiol. 1968 Jan;7(1):73–86. doi: 10.1111/j.1751-1097.1968.tb05831.x. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Johansen I. Incisions in ultraviolet irradiated circular bacteriophage lambda DNA molecules in excision proficient and deficient lysogens of E. coli. Mol Gen Genet. 1973;123(2):173–184. doi: 10.1007/BF00267333. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. The photochemistry, photobiology, and repair of polynucleotides. Prog Nucleic Acid Res Mol Biol. 1968;8:257–295. doi: 10.1016/s0079-6603(08)60548-6. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Anderson J. A., Barbour S. D. Excision repair properties of isogenic rec mutants of Escherichia coli K-12. J Bacteriol. 1972 Sep;111(3):723–730. doi: 10.1128/jb.111.3.723-730.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Evidence for the control by exrA and polA genes of two branches of the uvr gene-dependent excision repair pathway in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):175–182. doi: 10.1128/jb.116.1.175-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Involvement of DNA polymerase 3 in excision repair after ultraviolet irradiation. Nat New Biol. 1973 Aug 22;244(138):240–241. doi: 10.1038/newbio244240a0. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Van der Schueren E., Smith K. C. Separate branches of the uvr gene-dependent excision repair process in ultraviolet-irradiated Escherichia coli K-12 cells; their dependence upon growth medium and the polA, recA, recB, and exrA genes. J Bacteriol. 1974 Feb;117(2):717–725. doi: 10.1128/jb.117.2.717-725.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



