Abstract
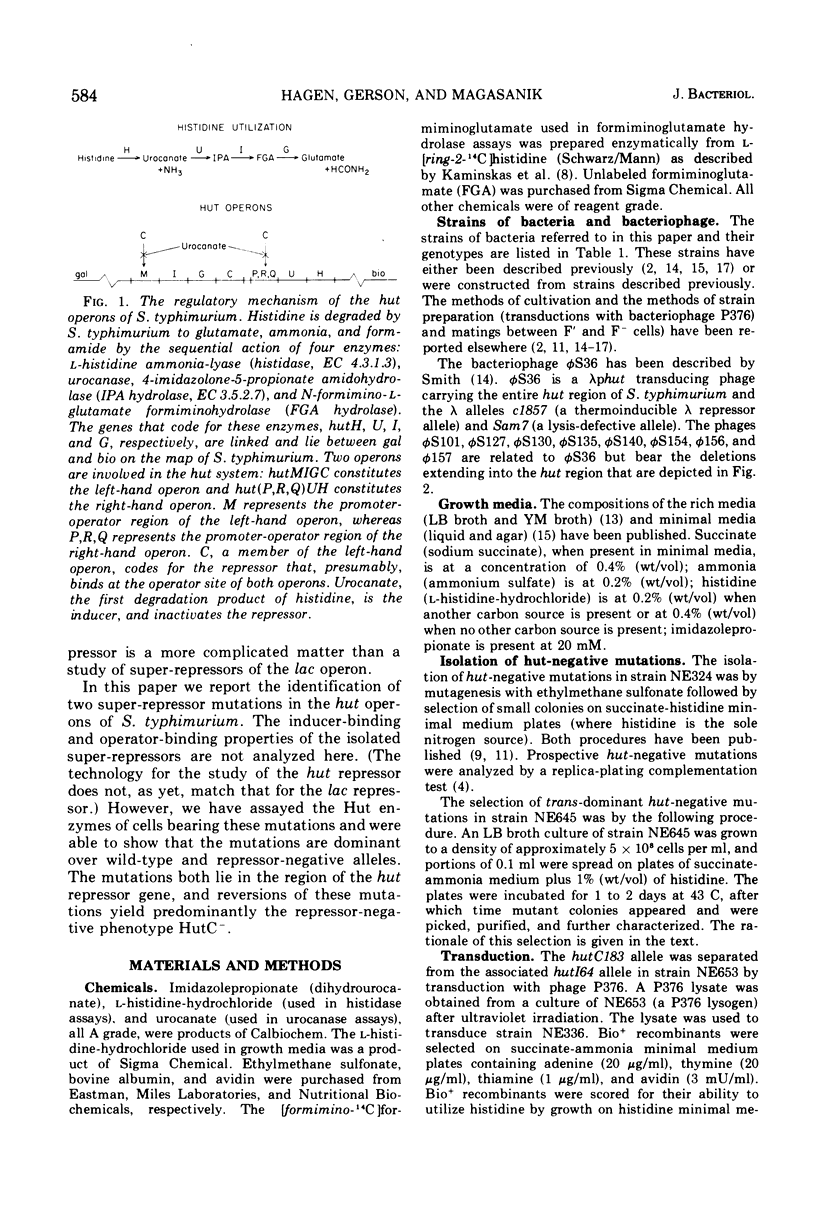
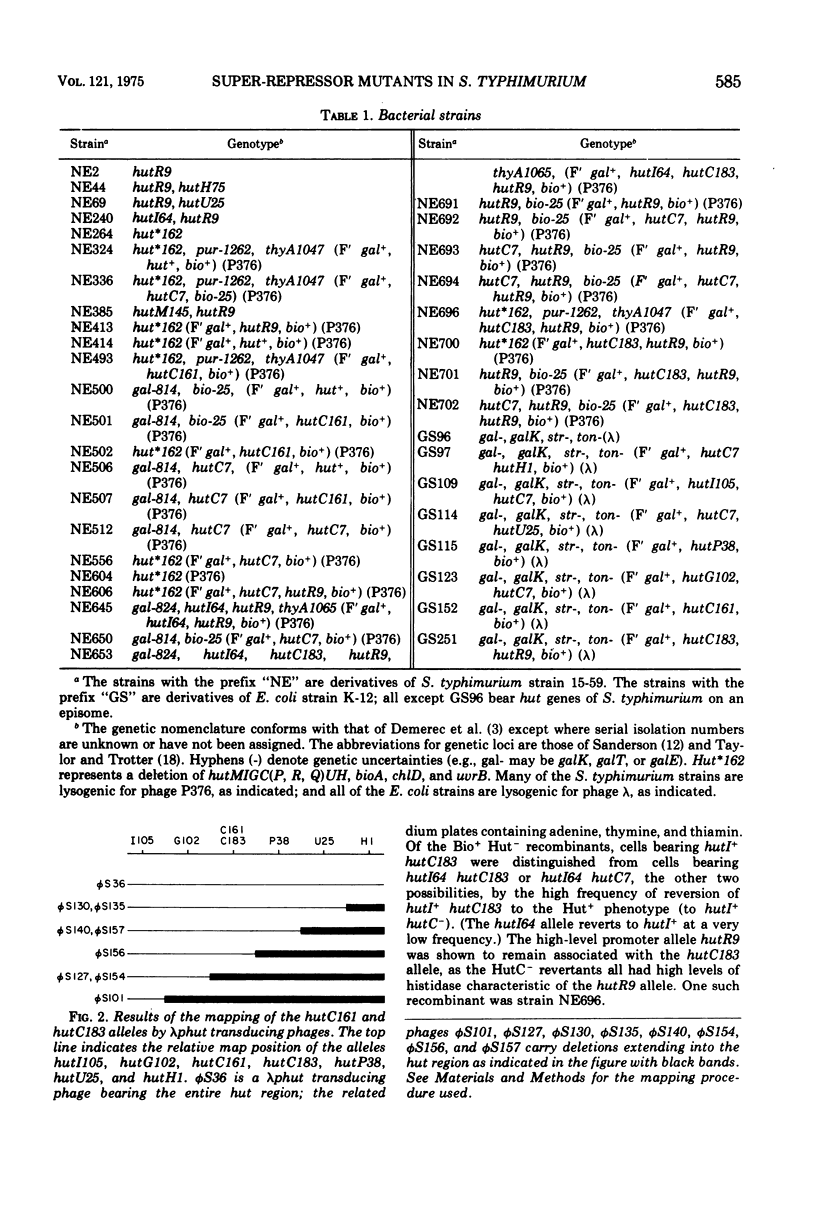
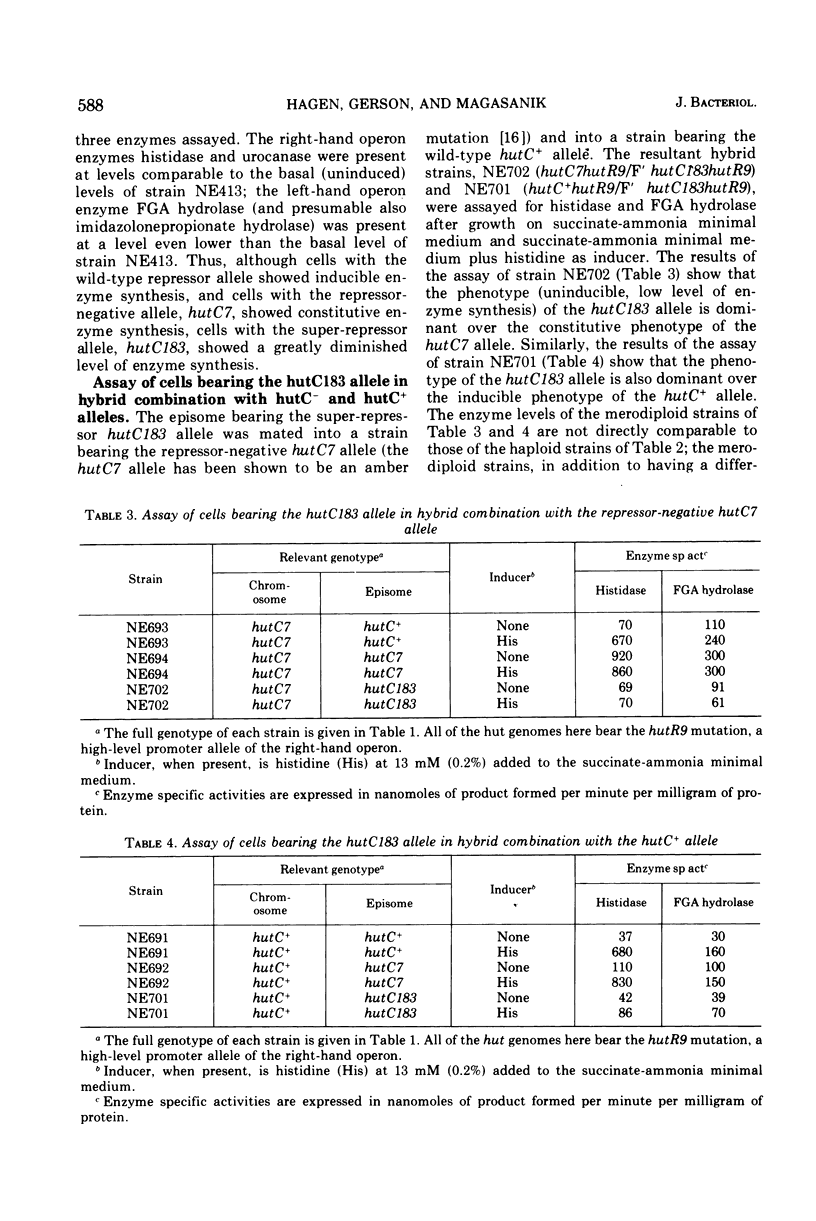
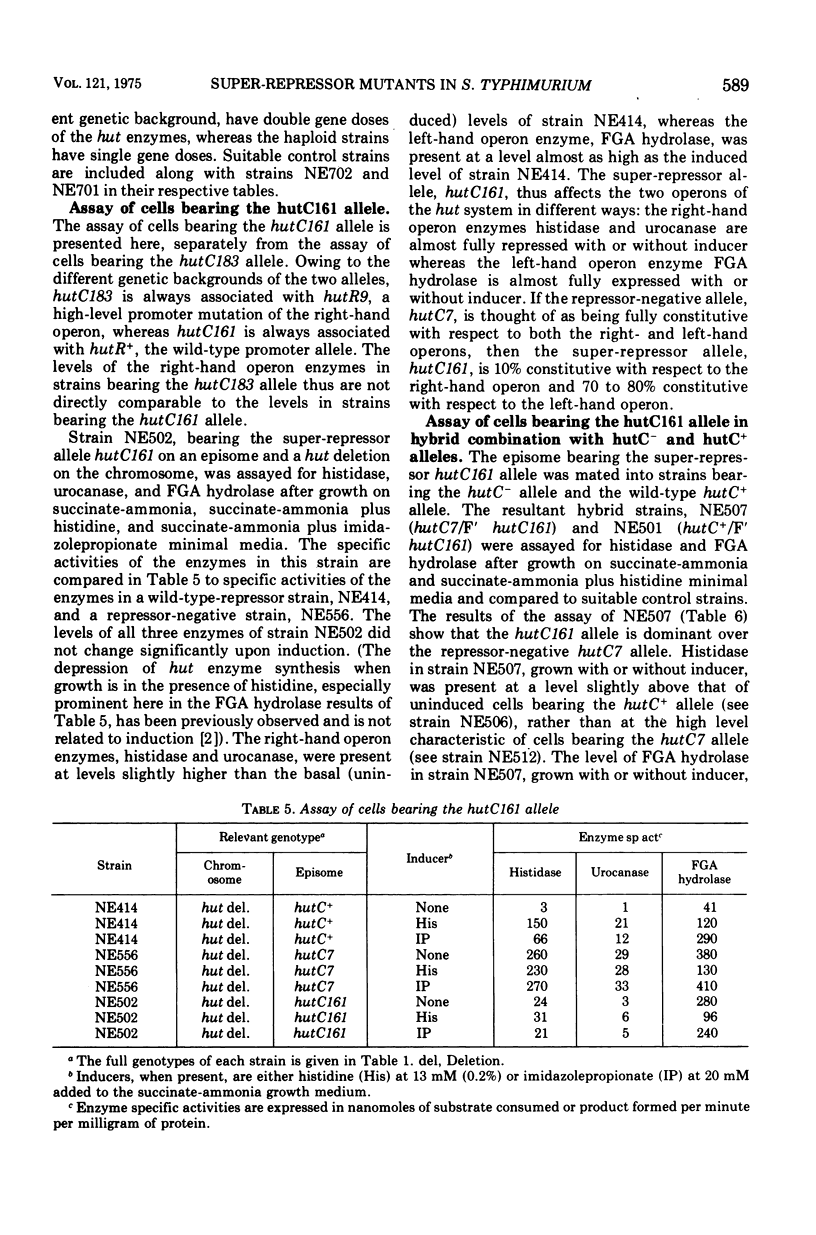
Two super-repressor mutations in the histidine utilization (hut) operons of Salmonella typhimurium are described. Cells bearing either of these mutations have levels of hut enzymes that do not increase above the uninduced levels when growth is in the presence of either histidine or the gratuitous inducer imidazole propionate. Both mutations lie in the region of the gene for the hut repressor, hutC, and reverse mutations of both are to the constitutive (repressor-negative) rather than to the inducible (wild type) phenotype. In hybrid merodiploid strains the super-repressor mutations are dominant over either wild-type (hutC+) or repressor-negative (hutC-) alleles. Whereas both super-repressor mutations cause the uninducible synthesis of hut enzymes, the degree of repression is different. One mutation causes repression of enzyme synthesis in one of the two hut operons to a level below the basal, uninduced level of wild-type cells. The other mutation causes repression to a lesser degree than in wild-type cells, so that the hut enzymes are present at a level above the normal basal level; this partially constitutive synthesis is greater for the enzymes of one of the hut operons than for the enzymes of the other. Thus, both mutations apparently result in repressors with altered operator-binding properties, in addition to altered inducer-binding properties.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill W. J., Magasanik B. Genetic and metabolic control of histidase and urocanase in Salmonella typhimurium, strain 15-59. J Biol Chem. 1969 Oct 10;244(19):5392–5402. [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen D. C., Lipton P. J., Magasanik B. Isolation of a trans-dominant histidase-negative mutant of Salmonella typhimurium. J Bacteriol. 1974 Nov;120(2):906–916. doi: 10.1128/jb.120.2.906-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen D. C., Magasanik B. Isolation of the self-regulated repressor protein of the Hut operons of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1973 Mar;70(3):808–812. doi: 10.1073/pnas.70.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe A., Riggs A. D., Bourgeois S. Lac repressor-operator interaction. V. Characterization of super- and pseudo-wild-type repressors. J Mol Biol. 1972 Feb 28;64(1):181–199. doi: 10.1016/0022-2836(72)90328-2. [DOI] [PubMed] [Google Scholar]
- Kaminskas E., Kimhi Y., Magasanik B. Urocanase and N-formimino-L-glutamate formiminohydrolase of Bacillus subtilis, two enzymes of the histidine degradation pathway. J Biol Chem. 1970 Jul 25;245(14):3536–3544. [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer E. R. On the control of lysogeny in phage lambda. Virology. 1970 Mar;40(3):624–633. doi: 10.1016/0042-6822(70)90207-2. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Halpern Y. S., Magasanik B. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. 4-imidazolone-5-propionate amidohydrolase and N-formimino-L-glutamate formiminohydrolase. J Biol Chem. 1971 May 25;246(10):3320–3329. [PubMed] [Google Scholar]
- Smith G. R., Magasanik B. Nature and self-regulated synthesis of the repressor of the hut operons in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1493–1497. doi: 10.1073/pnas.68.7.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Magasanik B. The two operons of the histidine utilization system in Salmonella typhimurium. J Biol Chem. 1971 May 25;246(10):3330–3341. [PubMed] [Google Scholar]
- Smith G. R. Specialized transduction of the Salmonella hut operons by coliphage lambda: deletion analysis of the hut operons employing lambda-phut. Virology. 1971 Jul;45(1):208–223. doi: 10.1016/0042-6822(71)90128-0. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLSON C., PERRIN D., COHN M., JACOB F., MONOD J. NON-INDUCIBLE MUTANTS OF THE REGULATOR GENE IN THE "LACTOSE" SYSTEM OF ESCHERICHIA COLI. J Mol Biol. 1964 Apr;8:582–592. doi: 10.1016/s0022-2836(64)80013-9. [DOI] [PubMed] [Google Scholar]


