Abstract
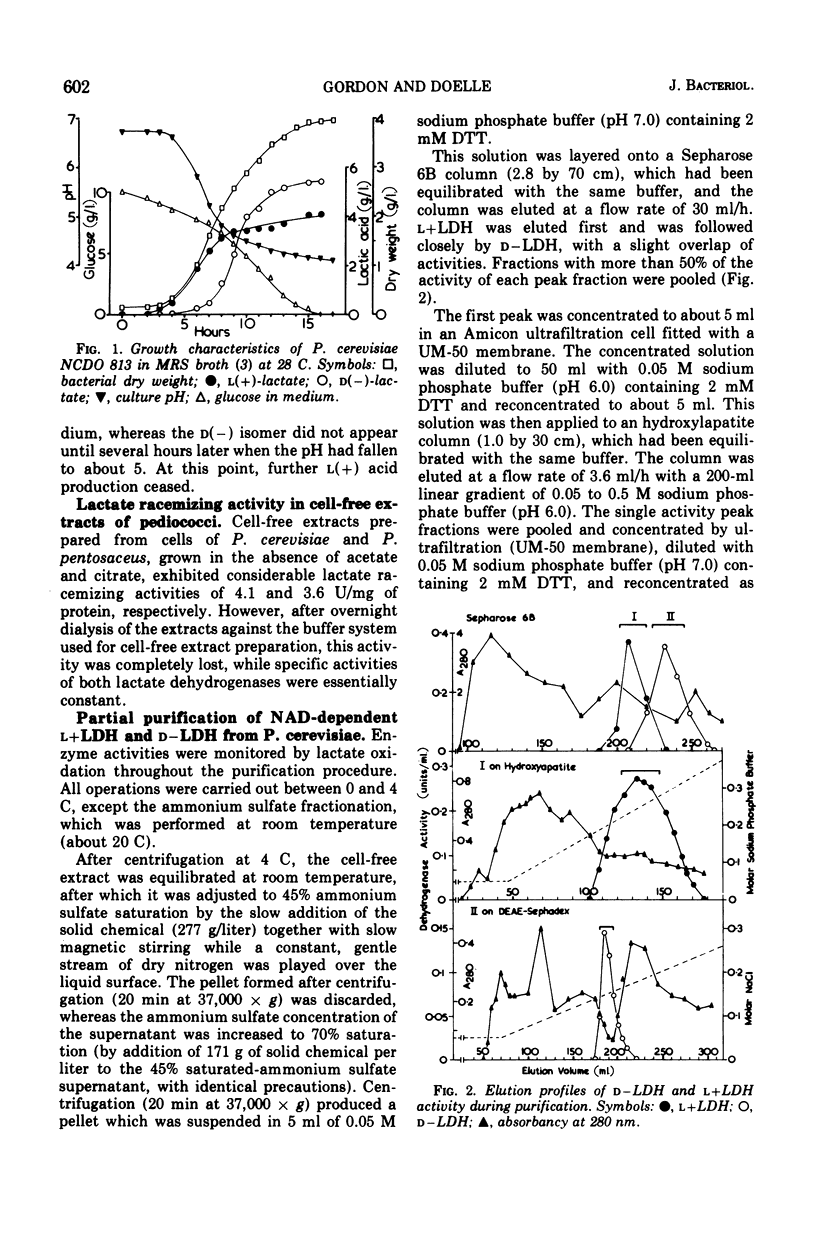
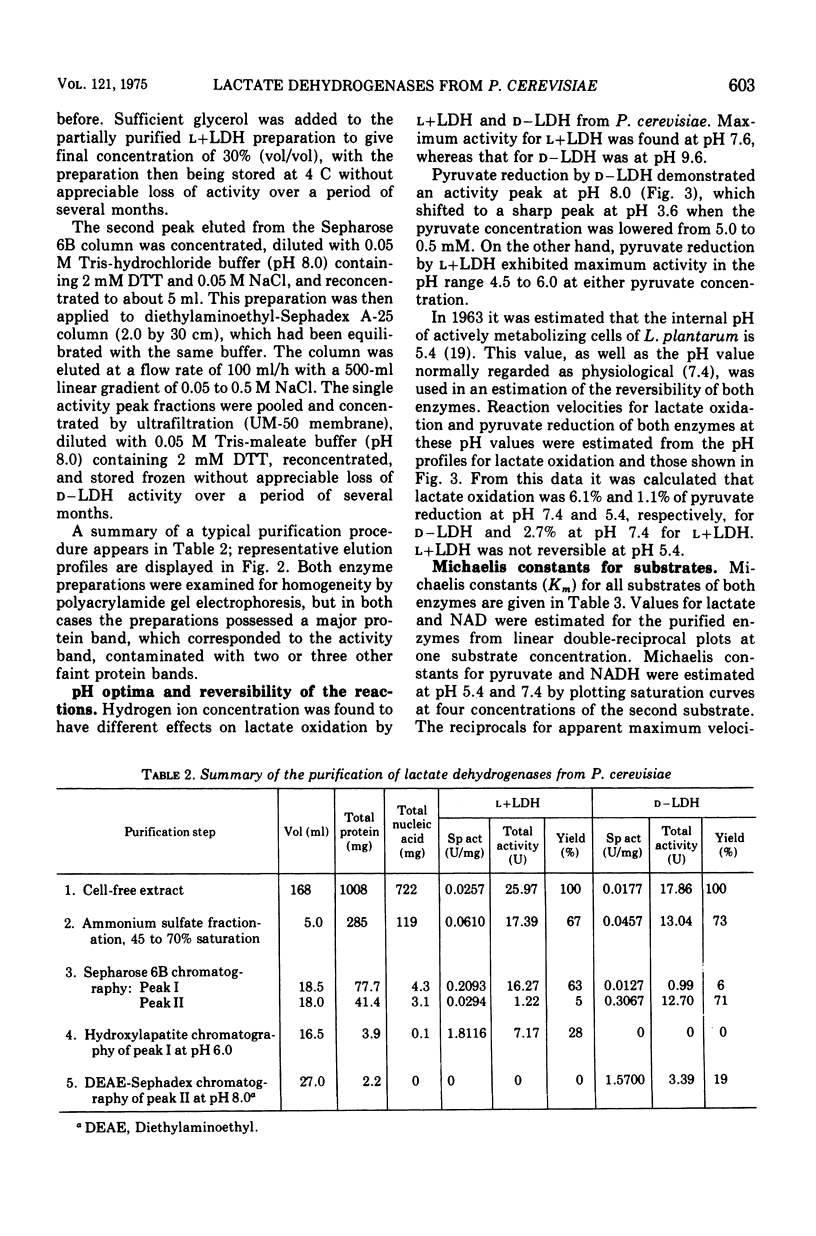
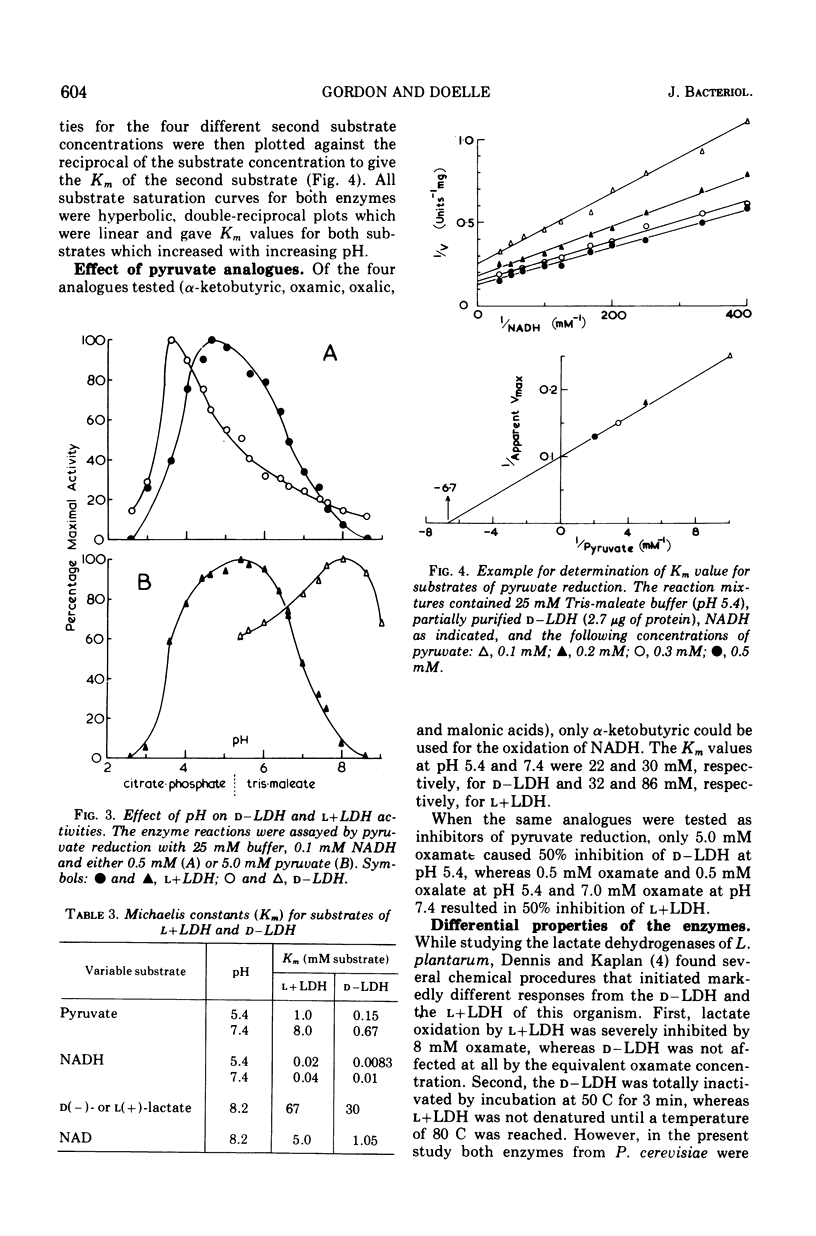
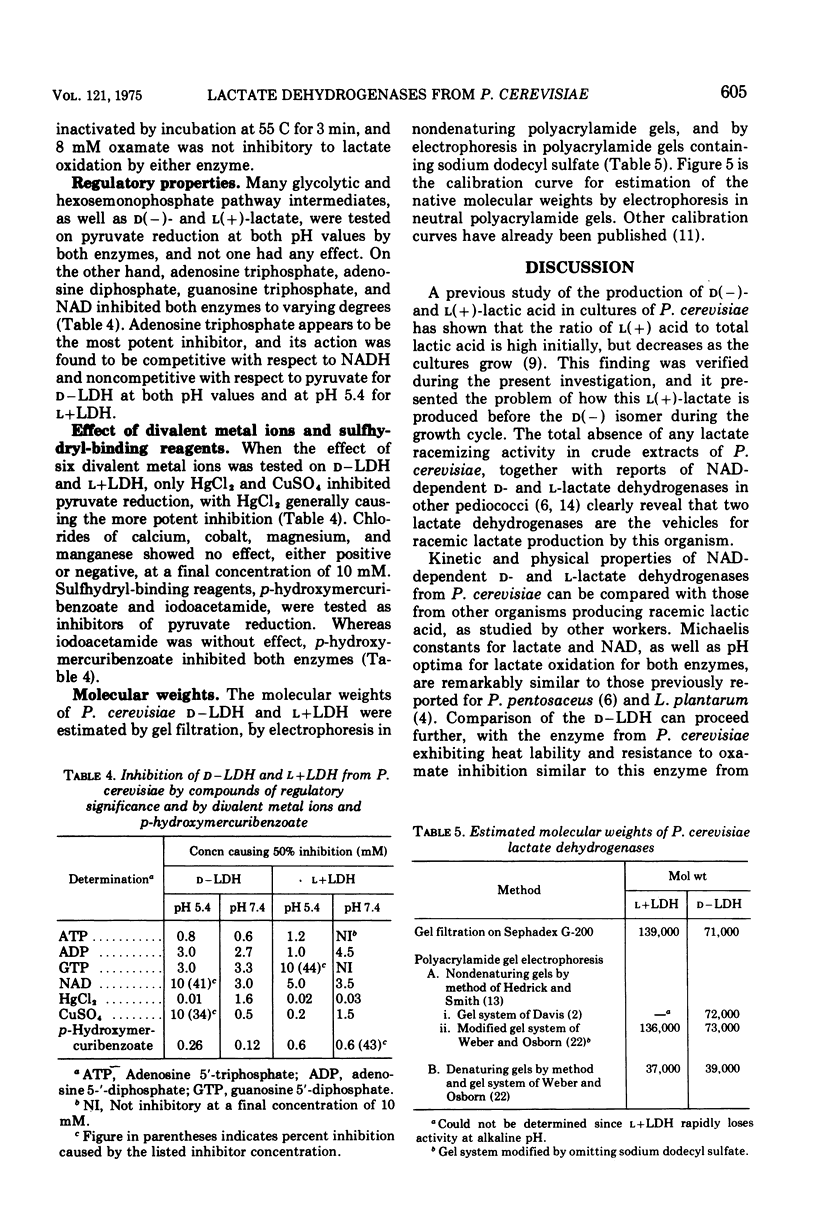
Nicotinamide adenine dinucleotide (NAD)-dependent d(minus)-and l(plus)-lactate dehydrogenases have been partially purified 89- and 70-fold simultaneously from cell-free extracts of Pediococcus cerevisiae. Native molecular weights, as estimated from molecular sieve chromatography and electrophoresis in nondenaturing polyacrylamide gels, are 71,000 to 73,000 for d(minus)-lactate dehydrogenase and 136,000 to 139,000 for l(plus)-lactate dehydrogenase. Electrophoresis in sodium dodecyl sulfate-containing gels reveals subunits with approximate molecular weights of 37,000 to 39,000 for both enzymes. By lowering the pyruvate concentration from 5.0 to 0.5 mM, the pH optimum for pyruvate reduction by d(minus)-lactate dehydrogenase decreases from pH 8.0 to 3.6. However, l(plus)-lactate dehydrogenase displays an optimum for pyruvate reduction between pH 4.5 and 6.0 regardless of the pyruvate concentration. The enzymes obey Michaelis-Menten kinetics for both pyruvate and reduced NAD at pH 5.4 and 7.4, with increased affinity for both substrates at the acid pH. alpha-Ketobutyrate can be used as a reducible substrate, whereas oxamate has no inhibitory effect on lactate oxidation by either enzyme. Adenosine triphosphate causes inhibition of both enzymes by competition with reduced NAD. Adenosine diphosphate is also inhibitory under the same conditions, whereas NAD acts as a product inhibitor. These results are discussed with relation to the lactate isomer production during the growth cycle of P. cerevisiae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DENNIS D., KAPLAN N. O. D- and L-lactic acid dehydrogenases in Lactobacillus plantarum. J Biol Chem. 1960 Mar;235:810–818. [PubMed] [Google Scholar]
- Dennis D., Reichlin M., Kaplan N. O. Lactic acid racemization. Ann N Y Acad Sci. 1965 Jul 31;119(3):868–876. doi: 10.1111/j.1749-6632.1965.tb47448.x. [DOI] [PubMed] [Google Scholar]
- Doelle H. W. Nicotinamide adenine dinucleotide-dependent and nicotinamide adenine dinucleotide-independent lactate dehydrogenases in homofermentative and heterofermentative lactic acid bacteria. J Bacteriol. 1971 Dec;108(3):1284–1289. doi: 10.1128/jb.108.3.1284-1289.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynon M. K., Jago G. R., Davidson B. E. The subunit structure of lactate dehydrogenase from Streptococcus cremoris US3. Eur J Biochem. 1972 Oct;30(2):348–353. doi: 10.1111/j.1432-1033.1972.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Garland R. C. Purification and properties of DL-lactate dehydrogenase from Leuconostoc mesenteroides. Arch Biochem Biophys. 1973 Jul;157(1):36–43. doi: 10.1016/0003-9861(73)90386-x. [DOI] [PubMed] [Google Scholar]
- Gasser F., Doudoroff M., Contopoulos R. Purification and properties of NAD-dependent lactic dehydrogenases of different species of lactobacillus. J Gen Microbiol. 1970 Aug;62(2):241–250. doi: 10.1099/00221287-62-2-241. [DOI] [PubMed] [Google Scholar]
- Gordon G. L., Doelle H. W. Molecular aspects for the metabolic regulation of the nicotinamide adenine dinucleotide-dependent D(-)-lactate dehydrogenase from Leuconostoc. Microbios. 1974 Mar-Apr;9(36):199–215. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Fukui S., Kitahara K. Purification and properties of lactate racemase from Lactobacillus sake. J Biochem. 1968 Jul;64(1):99–107. doi: 10.1093/oxfordjournals.jbchem.a128870. [DOI] [PubMed] [Google Scholar]
- Hontebeyrie M., Gasser F. Séparation et purification de la D-lactico-déshydrogénase et de la glucose-6-phosphate déshydrogénase de Leuconostoc lactis. Etude de quelques propriétés. Biochimie. 1973;55(9):1047–1056. doi: 10.1016/s0300-9084(73)80443-2. [DOI] [PubMed] [Google Scholar]
- MIZUSHIMA S., KITAHARA K. QUANTITATIVE STUDIES ON GLYCOLYTIC ENZYMES IN LACTOBACILLUS PLANTARUM. II. INTRACELLULAR CONCENTRATIONS OF GLYCOLYTIC INTERMEDIATES IN GLUCOSE-METABOLIZING WASHED CELLS. J Bacteriol. 1964 Jun;87:1429–1435. doi: 10.1128/jb.87.6.1429-1435.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZUSHIMA S., MACHIDA Y., KITAHARA K. QUANTITATIVE STUDIES ON GLYCOLYTIC ENZYMES IN LACTOBACILLUS PLANTARUM. I. CONCENTRATION OF INORGANIC IONS AND COENZYMES IN FERMENTING CELLS. J Bacteriol. 1963 Dec;86:1295–1300. doi: 10.1128/jb.86.6.1295-1300.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetter K. O., Kandler O. Untersuchungen zur Entstehung von DL-Milchsäure bei Lactobacillen und Charakterisierung einer Milchsäureracemase bei einigen Arten der Untergattung Streptobacterium. Arch Mikrobiol. 1973 Dec 31;94(3):221–247. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



