Abstract
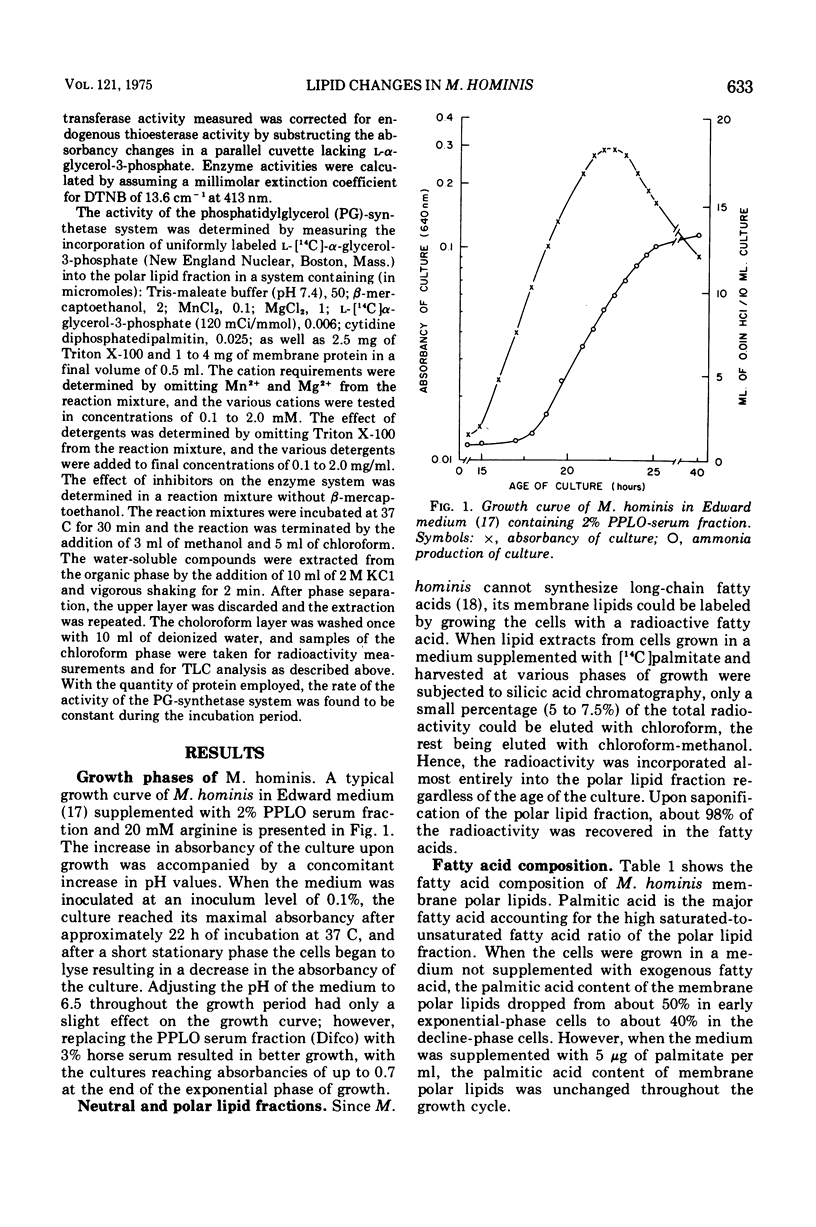
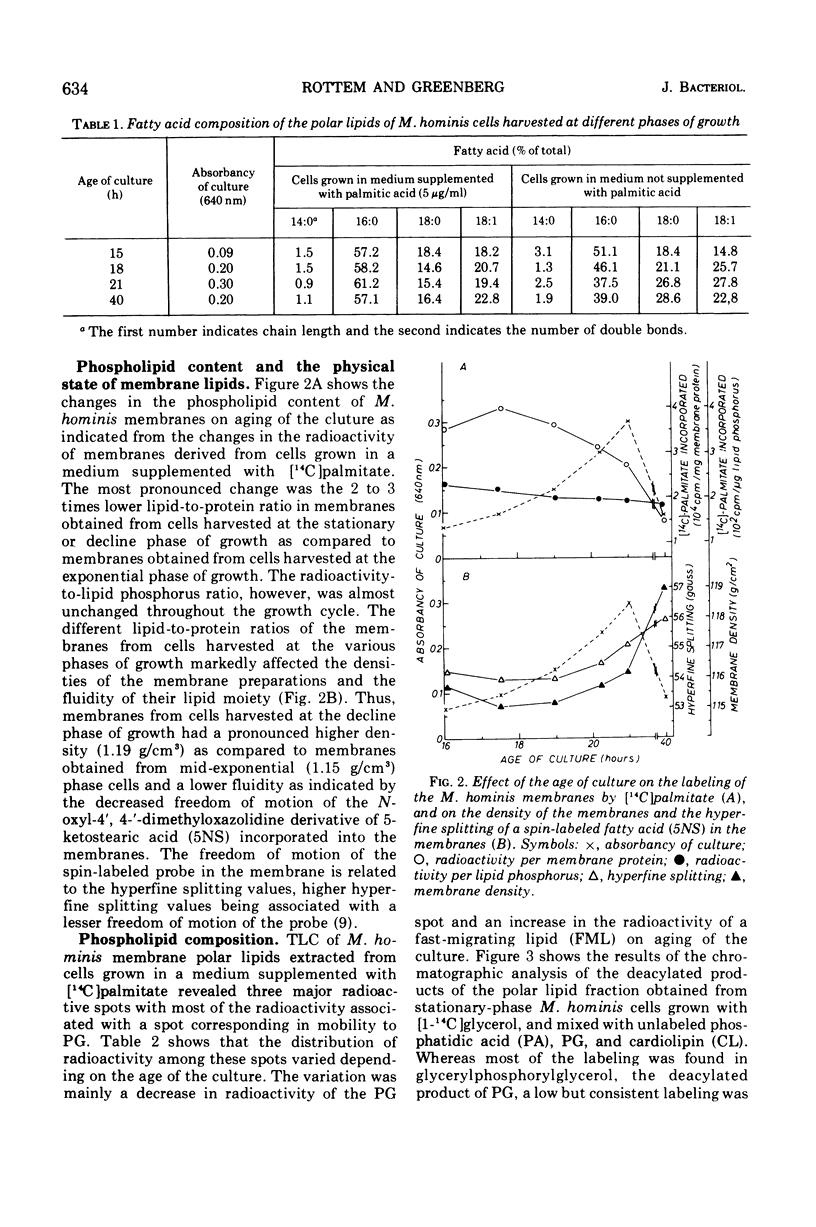
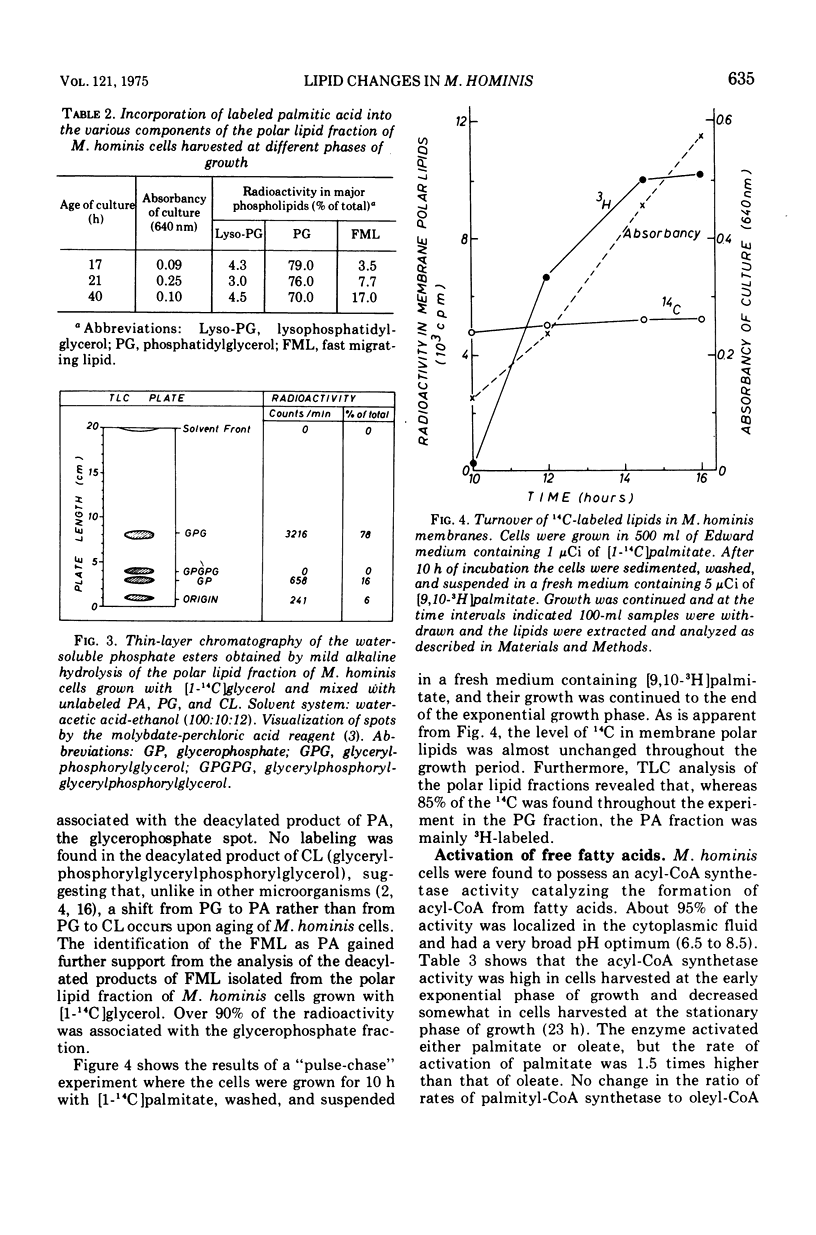
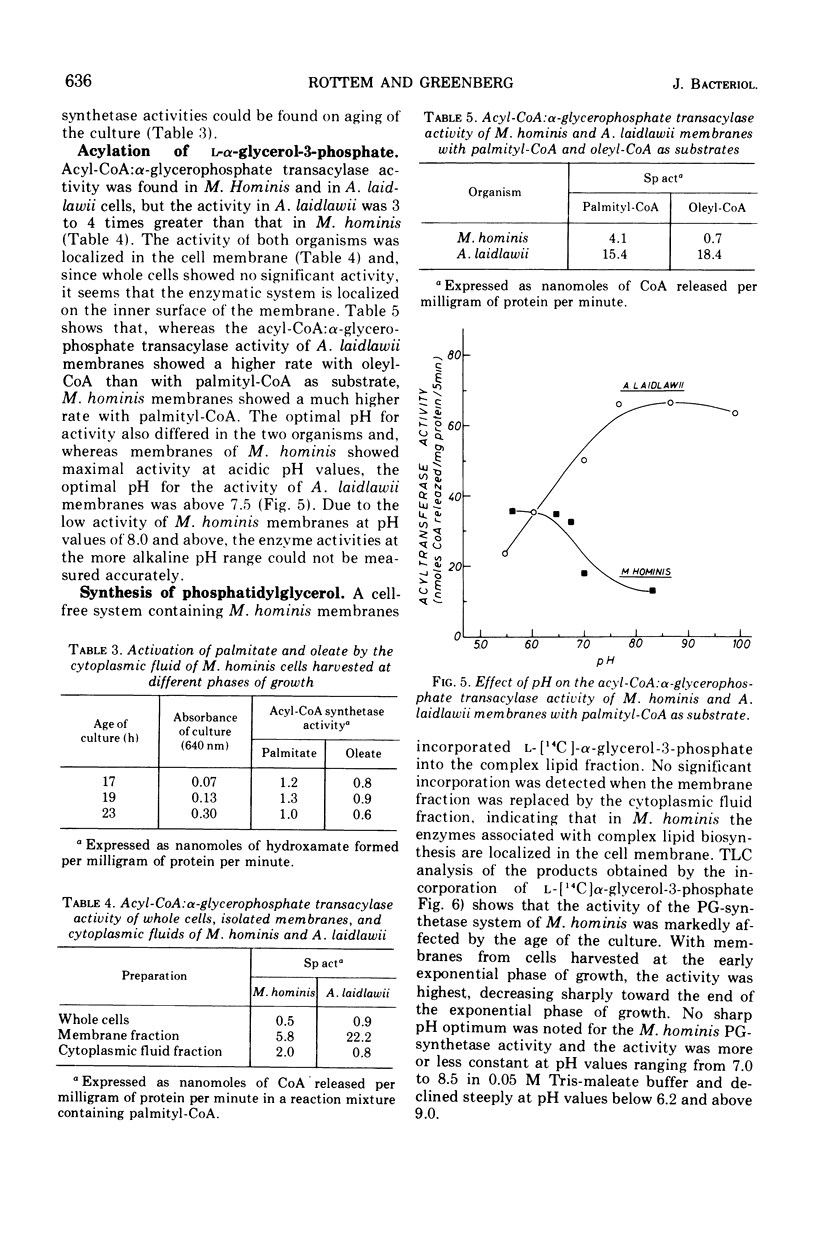
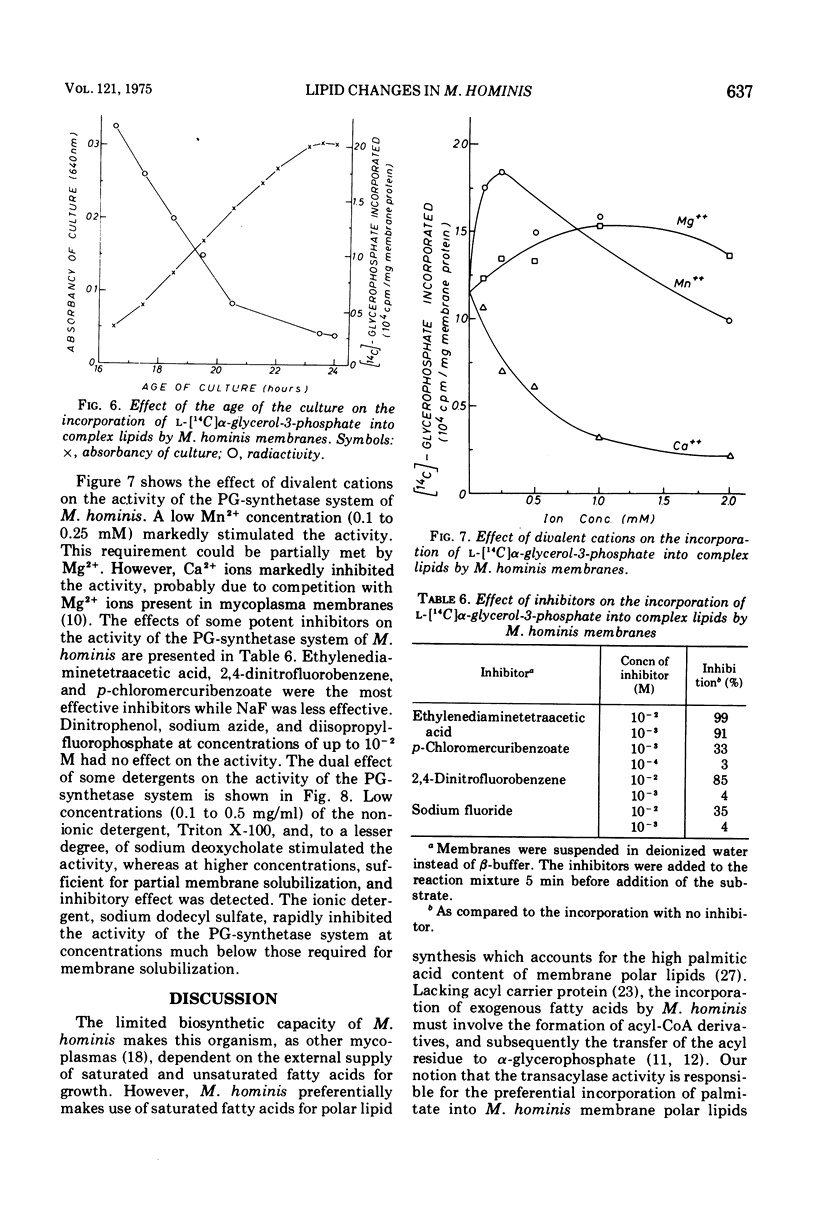
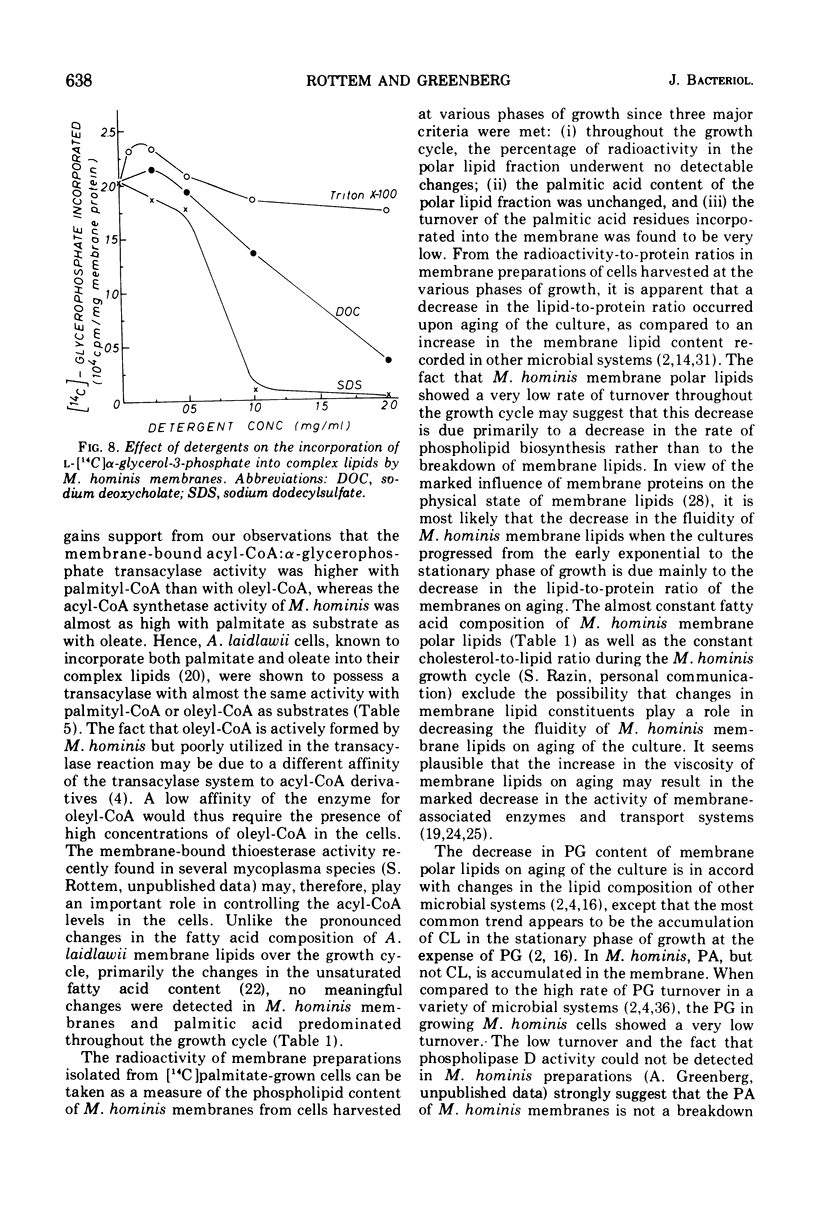
During the progression of Mycoplasma hominis cultures from the early logarithmic phase to the stationary phase of growth, the total phospholipid content of the cell membranes decreased. Measurement of the amount of the various phospholipids during the growth cycle showed that a decrease in the phosphatidylglycerol (PG) content, accompanied by an increase in the phosphatidic acid content, occurred upon aging of the culture. Pulse labeling experiments revealed that the PG, once formed, is relatively stable, undergoing no detectable turnover in growing cultures of M. hominis. No changes in the fatty acid composition of the membrane phospholipids were observed on aging of the culture, with palmitic acid predominating throughout the growth cycle. The preferential incorporation of palmitate into the phospholipid fraction is apparently caused by the higher activity of the membrane-bound acyl-coenzyme A (CoA):alpha-glycerophosphate transacylase with palmityl-CoA rather than with oleyl-CoA as substrate. The activity of the soluble acyl-CoA synthetase was the same whether palmitate or oleate served as substate. M. hominis membrane preparations contained a PG-synthetase system catalyzing the incorporation of L-alpha-glycerol-3-phosphate into PG. The activity of the PG synthetase system was markedly dependent on the age of the culture, being highest in cells from the early exponential phase of growth while declining sharply thereafter, and thus probably responsible, in part, for the decrease in PG content upon aging of the cells. Electron paramagnetic resonance spectra of a spin-labeled fatty acid incorporated in M. hominis membranes revealed a marked decrease in the freedom of motion of the spin label on aging of the culture. It is proposed that this decrease is due primarily to the decrease in the lipid-to-protein ratio of the membranes and has a marked effect on the activity of membrane-associated enzymes and transport systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Card G. L. Metabolism of phosphatidylglycerol, phosphatidylethanolamine, and cardiolipin of Bacillus stearothermophilus. J Bacteriol. 1973 Jun;114(3):1125–1137. doi: 10.1128/jb.114.3.1125-1137.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Orientation and motion of amphiphilic spin labels in membranes. Proc Natl Acad Sci U S A. 1969 Sep;64(1):20–27. doi: 10.1073/pnas.64.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kahane I., Ne'eman Z., Razin S. Divalent cations in native and reaggregated mycoplasma membranes. J Bacteriol. 1973 Feb;113(2):666–671. doi: 10.1128/jb.113.2.666-671.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall C. L., Brown A. D. The membrane lipids of Halobacterium halobium. Biochem J. 1968 Dec;110(3):441–448. doi: 10.1042/bj1100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Pollack M. E., Cleverdon R. C. Fractionation of mycoplasma cells for enzyme localization. Life Sci. 1965 May;4(9):973–977. doi: 10.1016/0024-3205(65)90200-6. [DOI] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Razin S., Gottfried L., Rottem S. Amino acid transport in Mycoplasma. J Bacteriol. 1968 May;95(5):1685–1691. doi: 10.1128/jb.95.5.1685-1691.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- Razin S., Tourtellotte M. E., McElhaney R. N., Pollack J. D. Influence of lipid components of Mycoplasma laidlawii membranes on osmotic fragility of cells. J Bacteriol. 1966 Feb;91(2):609–616. doi: 10.1128/jb.91.2.609-616.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Muhsam-Peled O., Razin S. Acyl carrier protein in mycoplasmas. J Bacteriol. 1973 Feb;113(2):586–591. doi: 10.1128/jb.113.2.586-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Isolation of mycoplasma membranes by digitonin. J Bacteriol. 1972 May;110(2):699–705. doi: 10.1128/jb.110.2.699-705.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Membrane lipids of Mycoplasma hominis. J Bacteriol. 1973 Feb;113(2):565–571. doi: 10.1128/jb.113.2.565-571.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Sugar transport in Mycoplasma gallisepticum. J Bacteriol. 1969 Feb;97(2):787–792. doi: 10.1128/jb.97.2.787-792.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Samuni A. Effect of proteins on the motion of spin-labeled fatty acids in mycoplasma membranes. Biochim Biophys Acta. 1973 Feb 27;298(1):32–38. doi: 10.1016/0005-2736(73)90006-0. [DOI] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., BAKAY B., CONOVER M. J., TOENNIES G. Protoplast membrane of Streptococcus faecalis. J Bacteriol. 1963 Jan;85:168–176. doi: 10.1128/jb.85.1.168-176.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANLEY C. W. THIN-LAYER CHROMATOGRAPHY OF ORGANOPHOSPHORUS PESTICIDES AND ACIDS ON MICROCHROMATOPLATES. J Chromatogr. 1964 Dec;16:467–475. doi: 10.1016/s0021-9673(01)82517-6. [DOI] [PubMed] [Google Scholar]
- STERN I., SHAPIRO B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953 May;6(2):158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N., Smith P. F., Koostra W. L. The lipid composition of Mycoplasma laidlawii strain B. Biochem J. 1968 Apr;107(3):329–333. doi: 10.1042/bj1070329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Temperature control of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1971 May;106(2):449–455. doi: 10.1128/jb.106.2.449-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]
- de Kruyff B., van Dijck P. W., Godlbach R. W., Demel R. A., van Deenen L. L. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Dec 22;330(3):269–282. doi: 10.1016/0005-2736(73)90232-0. [DOI] [PubMed] [Google Scholar]


