Abstract
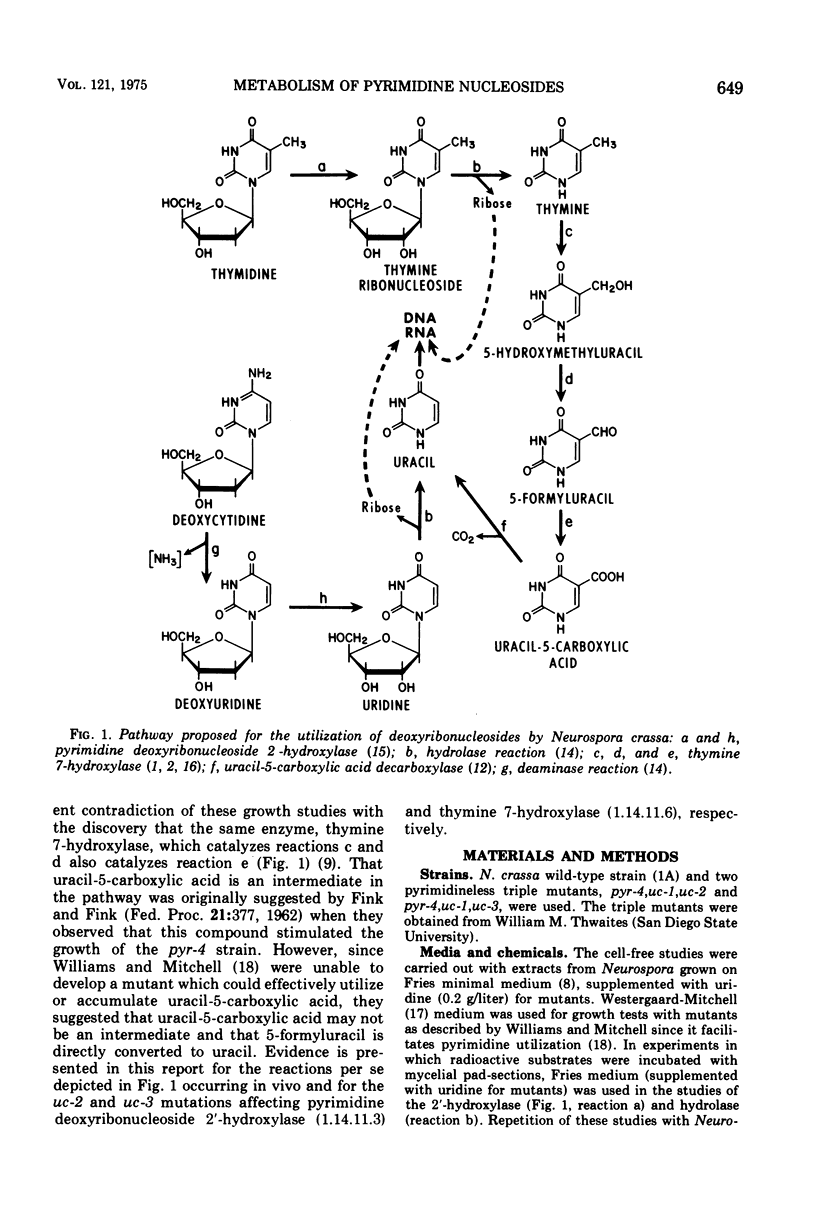
The experiments in this report involve the following series of reactions which were previously demonstrated with purified enzyme preparations from Neurospora crassa: thymidine a yields thymine ribonucleoside b yields thymine c yields 5-hydroxymethyluracil d yields 5-formyluracil e yields uracil-5-carboxylic acid f yields uracil. The evidence for some of the reactions occurring in vivo has been incomplete and for others totally lacking. In this paper intact cells of Neurospora are shown to be capable of converting the substrates of each of the reactions to the corresponding products. Studies are described which were carried out in vivo and in vitro with the pyrimidineless strains pyr-4,uc-1,uc-2 and pyr-4,uc-1,uc-3, developed by Williams and Mitchell. The results reported in the present paper indicate that (reaction a) and the uc-3 mutation affects thymine 7-hydroxylase (reactions c,d, and e). Evidence is presented for the 2'-hydroxylase reaction being the major, if not only, way by which Neurospora can initiate the conversion of thymidine to the pyrimidines of nucleic acids and for the 2'-hydroxylation of thymidine and deoxyuridine being catalyzed by the same enzyme. Deoxycytidine was shown not to be hydroxylated in intact cells but instead deaminated to deoxyuridine, which in turn was converted to uridine. Further studies with the uc-3-carrying strain showed that an enzyme other than thymine 7-hydroxylase can also convert 5-formyluracil to uracil-5-carboxylic acid.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT M. T., KADNER R. J., FINK R. M. CONVERSION OF THYMINE TO 5-HYDROXYMETHYLURACIL IN A CELL-FREE SYSTEM. J Biol Chem. 1964 Jan;239:156–159. [PubMed] [Google Scholar]
- Abbott M. T., Dragila T. A., McCroskey R. P. The formation of 5-formyluracil by cell-free preparations from Neurospora crassa. Biochim Biophys Acta. 1968 Nov 20;169(1):1–6. doi: 10.1016/0005-2787(68)90002-6. [DOI] [PubMed] [Google Scholar]
- FINK R. M., FINK K. Biosynthesis of radioactive RNA and DNA pyrimidines from thymidine-2-C-14. Biochem Biophys Res Commun. 1961 Oct 23;6:7–10. doi: 10.1016/0006-291x(61)90174-7. [DOI] [PubMed] [Google Scholar]
- FINK R. M., FINK K. Relative retention of H3 and C14 labels of nucleosides incorporated into nucleic acids of Neurospora. J Biol Chem. 1962 Sep;237:2889–2891. [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Liu C. K., Hsu C. A., Abbott M. T. Catalysis of three sequential dioxygenase reactions by thymine 7-hydroxylase. Arch Biochem Biophys. 1973 Nov;159(1):180–187. doi: 10.1016/0003-9861(73)90443-8. [DOI] [PubMed] [Google Scholar]
- Liu C. K., Shaffer P. M., Slaughter R. S., McCroskey R. P., Abbott M. T. Stoichiometry of the pyrimidine deoxyribonucleoside 2'-hydroxylase reaction and of the conversions of 5-hydroxymethyluracil to 5-formyluracil and of the latter to uracil-5-carboxylic acid. Biochemistry. 1972 May 23;11(11):2172–2176. doi: 10.1021/bi00761a025. [DOI] [PubMed] [Google Scholar]
- McCroskey R. P., Griswold W. R., Sokoloff R. L., Sevier E. D., Lin S., Liu K., Shaffer P. M., Palmatier R. D., Parker T. S., Abbott M. T. Studies pertaining to the purification and properties of thymine 7-hydroxylase. Biochim Biophys Acta. 1971 Feb 10;227(2):264–277. doi: 10.1016/0005-2744(71)90059-3. [DOI] [PubMed] [Google Scholar]
- Palmatier R. D., McCroskey R. P., Abbott M. T. The enzymatic conversion of uracil 5-carboxylic acid to uracil and carbon dioxide. J Biol Chem. 1970 Dec 25;245(24):6706–6710. [PubMed] [Google Scholar]
- Shaffer P. M., McCroskey R. P., Abbott M. T. Substrate specificity of the hydroxylase reaction in which thymidine is converted to thymine ribonucleoside. Biochim Biophys Acta. 1972 Feb 28;258(2):387–394. doi: 10.1016/0005-2744(72)90230-6. [DOI] [PubMed] [Google Scholar]
- Shaffer P. M., McCroskey R. P., Palmatier R. D., Midgett R. J., Abbott M. T. The cell-free conversion of a deoxyribonucleoside to a ribonucleoside without detachment of the deoxyribose. Biochem Biophys Res Commun. 1968 Dec 9;33(5):806–811. doi: 10.1016/0006-291x(68)90232-5. [DOI] [PubMed] [Google Scholar]
- Watanabe M. S., McCroskey R. P., Abbott M. T. The enzymatic conversion of 5-formyluracil to uracil 5-carboxylic acid. J Biol Chem. 1970 Apr 25;245(8):2023–2026. [PubMed] [Google Scholar]
- Williams L. G., Mitchell H. K. Mutants affecting thymidine metabolism in Neurospora crassa. J Bacteriol. 1969 Oct;100(1):383–389. doi: 10.1128/jb.100.1.383-389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


